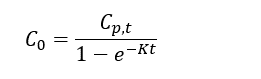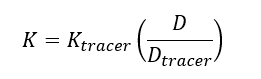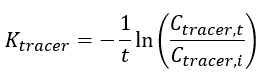User:Jhurley/sandbox
Sediment Porewater Dialysis Passive Samplers for Inorganics (Peepers)
Sediment porewater dialysis passive samplers, also known as “peepers,” are sampling devices that allow the measurement of dissolved inorganic ions in the porewater of a saturated sediment. Peepers function by allowing freely-dissolved ions in sediment porewater to diffuse across a micro-porous membrane towards water contained in an isolated compartment that has been inserted into sediment. Once retrieved after a deployment period, the resulting sample obtained can provide concentrations of freely-dissolved inorganic constituents in sediment, which provides measurements that can be used for understanding contaminant fate and risk. Peepers can also be used in the same manner in surface water, although this article is focused on the use of peepers in sediment.
Contents
- 1 Sediment Porewater Dialysis Passive Samplers for Inorganics (Peepers)
- 2 Introduction
- 3 Peeper Designs
- 4 Peepers Preparation, Deployment and Retrieval
- 5 Equilibrium Determination (Tracers)
- 6 Using Peeper Data at a Sediment Site
- 7 Extraction Methods
- 8 HPLC-UV and HPLC-MS Methods
- 9 Summary
- 10 References
- 11 See Also
Related Article(s):
- Contaminated Sediments - Introduction
- Contaminated Sediment Risk Assessment
- In Situ Treatment of Contaminated Sediments with Activated Carbon
- Passive Sampling of Munitions Constituents
- Sediment Capping
- Mercury in Sediments
- Passive Sampling of Sediments
Contributor(s):
- Florent Risacher, M.Sc.
- Jason Conder, Ph.D.
Key Resource(s):
- A review of peeper passive sampling approaches to measure the availability of inorganics in sediment porewater[1]
- Best Practices User’s Guide: Standardizing Sediment Porewater Passive Samplers for Inorganic Constituents of Concern[2]
Introduction
Biologically available inorganic constituents associated with sediment toxicity can be quantified by measuring the freely-dissolved fraction of contaminants in the porewater[3][4]. Classical sediment porewater analysis usually consists of collecting large volumes of bulk sediments which are then mechanically squeezed or centrifuged to produce a supernatant, or suction of porewater from intact sediment, followed by filtration and collection[5]. The extraction and measurement processes present challenges due to the heterogeneity of sediments, physical disturbance, high reactivity of some complexes, and interaction between the solid and dissolved phases, which can impact the measured concentration of dissolved inorganics[6]. For example, sampling disturbance can affect redox conditions[7][8], which can lead to under or over representation of inorganic chemical concentrations relative to the true dissolved phase concentration in the sediment porewater[9][5].
To address the complications with mechanical porewater sampling, passive sampling approaches for inorganics have been developed to provide a method that has a low impact on the surrounding geochemistry of sediments and sediment porewater, thus enabling more precise measurements of inorganics[4]. Sediment porewater dialysis passive samplers, also known as “peepers,” were developed more than 45 years ago[10] and refinements to the method such as the use of reverse tracers have been made, improving the acceptance of the technology as decision-making tool.
Peeper Designs
Peepers (Figure 1) are inert containers with a small volume (typically 1-100 mL) of purified water (“peeper water”) capped with a semi-permeable membrane. Peepers can be manufactured in a wide variety of formats (Figure 2, Figure 3) and deployed in in various ways.
Two designs are commonly used for peepers. Frequently, the designs are close adaptations of the original multi-chamber Hesslein design[10] (Figure 2), which consists of an acrylic sampler body with multiple sample chambers machined into it. Peeper water inside the chambers is separated from the outside environment by a semi-permeable membrane, which is held in place by a top plate fixed to the sampler body using bolts or screws. An alternative design consists of single-chamber peepers constructed using a single sample vial with a membrane secured over the mouth of the vial, as shown in Figure 3, and applied in Teasdale et al.[7], Serbst et al.[11], Thomas and Arthur[12], Passeport et al.[13], and Risacher et al.[2]. The vial is filled with deionized water, and the membrane is held in place using the vial cap or an o-ring. Individual vials are either directly inserted into sediment or are incorporated into a support structure to allow multiple single-chamber peepers to be deployed at once over a given depth profile (Figure 3).
Peepers Preparation, Deployment and Retrieval
Peepers are often prepared in laboratories but are also commercially available in a variety of designs from several suppliers. Peepers are prepared by first cleaning all materials to remove even trace levels of metals before assembly. The water contained inside the peeper is sometimes deoxygenated, and in some cases the peeper is maintained in a deoxygenated atmosphere until deployment[14]. However, recent studies[2] have shown that deoxygenation prior to deployment does not significantly impact sampling results due to oxygen rapidly diffusing out of the peeper during deployment. Once assembled, peepers are usually shipped in a protective bag inside a hard-case cooler for protection.
Peepers are deployed by insertion into sediment for a period of a few days to a few weeks. Insertion into the sediment can be achieved by wading to the location when the water depth is shallow, by using push poles for deeper deployments[2], or by professional divers for the deepest sites. If divers are used, an appropriate boat or ship will be required to accommodate the diver and their equipment. Whichever method is used, peepers should be attached to an anchor or a small buoy to facilitate retrieval at the end of the deployment period.
During deployment, passive sampling is achieved via diffusion, as the enclosed volume of peeper water equilibrates with the surrounding sediment porewater via transport of inorganics through the peeper’s semi-permeable membrane (Figure 4). It is assumed that the peeper insertion does not greatly alter geochemical conditions that affect freely-dissolved inorganics. Additionally, it is assumed that the peeper water equilibrates with freely-dissolved inorganics in sediment in such a way that the concentration of inorganics in the peeper water would be equal to that of the concentration of inorganics in the sediment porewater.
After retrieval, the peepers are brought to the surface and usually preserved until they can be processed. This can be achieved by storing the peepers inside a sealable, airtight bag with either inert gas or oxygen absorbing packets[2]. The peeper water can then be processed by quickly pipetting it into an appropriate sample bottle which usually contains a preservative (e.g., nitric acid for metals). This step is generally conducted in the field. Samples are stored on ice to maintain a temperature of less than 4°C and shipped to an analytical laboratory. The samples are then analyzed for inorganics by standard methods (i.e., USEPA SW-846). The results obtained from the analytical laboratory are then used directly or assessed using the equations below if a reverse tracer is used because deployment time is insufficient for all analytes to reach equilibrium.
Equilibrium Determination (Tracers)
The equilibration period of peepers can last several weeks and depends on deployment conditions, analyte of interest, and peeper design. In many cases, it is advantageous to use pre-equilibrium methods that can use measurements in peepers deployed for shorter periods to predict concentrations at equilibrium[15].
Although the equilibrium concentration of an analyte in sediment can be evaluated by examining analyte results for peepers deployed for several different amounts of time (i.e., a time series), this is impractical for typical field investigations because it would require several mobilizations to the site to retrieve samplers. Alternately, reverse tracers (referred to as a performance reference compound when used with organic compound passive sampling) can be used to evaluate the percentage of equilibrium reached by a passive sampler.
Thomas and Arthur[12] studied the use of a reverse tracer to estimate percent equilibrium in lab experiments and a field application. They concluded that bromide can be used to estimate concentrations in porewater using measurements obtained before equilibrium is reached. Further studies were also conducted by Risacher et al.[2] showed that lithium can also be used as a tracer for brackish and saline environments. Both studies included a mathematical model for estimating concentrations of ions in external media (C0) based on measured concentrations in the peeper chamber (Cp,t), the elimination rate of the target analyte (K) and the deployment time (t):
The elimination rate of the target analyte (K) is calculated using Equation 2:
The elimination rate of the tracer (Ktracer) is calculated using Equation 3:
Using this set of equations allows the calculation of the porewater concentration of the analyte at equilibrium. A template for these calculations can be found in appendix of Risacher et al.[2]
Using Peeper Data at a Sediment Site
| Compound | Acronym | CAS Number |
|---|---|---|
| 1,2-Dinitrobenzene (surrogate) | 1,2-DNB (surr.) | 528-29-0 |
| 1,3-Dinitrobenzene | 1,3-DNB | 99-65-0 |
| 1,3,5-Trinitrobenzene | 1,3,5-TNB | 99-35-4 |
| 1,4-Dinitrobenzene | 1,4-DNB (surr.) | 100-25-4 |
| 2-Amino-4,6-dinitrotoluene | 2-Am-4,6-DNT | 35572-78-2 |
| 2-Nitrophenol | 2-NP | 88-75-5 |
| 2-Nitrotoluene | 2-NT | 88-72-2 |
| 2,4-Dinitrophenol | 2,4-DNP | 51-28-5 |
| 2,4-Dinitrotoluene | 2,4-DNT | 121-14-2 |
| 2,4,6-Trinitrophenol | Picric Acid (PA) | 88-89-1 |
| 2,4,6-Trinitrotoluene | 2,4,6-TNT | 118-96-7 |
| 2,6-Dinitrotoluene | 2,6-DNT | 606-20-2 |
| 3-Nitrotoluene | 3-NT | 99-08-1 |
| 3,5-Dinitroaniline | 3,5-DNA | 618-87-1 |
| 4-Amino-2,6-dinitrotoluene | 4-Am-2,6-DNT | 19406-51-0 |
| 4-Nitrophenol | 4-NP | 100-02-7 |
| 4-Nitrotoluene | 4-NT | 99-99-0 |
| 2,4-Dinitroanisole | DNAN | 119-27-7 |
| Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine | HMX | 2691-41-0 |
| Nitrobenzene | NB | 98-95-3 |
| Nitroglycerine | NG | 55-63-0 |
| Nitroguanidine | NQ | 556-88-7 |
| 3-Nitro-1,2,4-triazol-5-one | NTO | 932-64-9 |
| ortho-Nitrobenzoic acid | o-NBA (surr.) | 552-16-9 |
| Pentaerythritol tetranitrate | PETN | 78-11-5 |
| Hexahydro-1,3,5-trinitro-1,3,5-triazine | RDX | 121-82-4 |
| N-Methyl-N-(2,4,6-trinitrophenyl)nitramide | Tetryl | 479-45-8 |
| Note: Analytes in bold are not identified by EPA Method 8330B. | ||
The primary intention of the analytical methods presented here is to support the monitoring of legacy and insensitive munitions contamination on test and training ranges, however legacy and insensitive munitions often accompany each other at demilitarization facilities, manufacturing facilities, and other environmental sites. Energetic materials typically appear on ranges as small, solid particulates and due to their varying functional groups and polarities, can partition in various environmental compartments[16]. To ensure that contaminants are monitored and controlled at these sites and to sustainably manage them a variety of sample matrices (surface or groundwater, process waters, soil, and tissues) must be considered. (Process water refers to water used during industrial manufacturing or processing of legacy and insensitive munitions.) Furthermore, additional analytes must be added to existing methodologies as the usage of IM compounds changes and as new degradation compounds are identified. Of note, relatively new IM formulations containing NTO, DNAN, and NQ are seeing use in IMX-101, IMX-104, Pax-21 and Pax-41 (Table 1)[17][18].
Sampling procedures for legacy and insensitive munitions are identical and utilize multi-increment sampling procedures found in USEPA Method 8330B Appendix A[19]. Sample hold times, subsampling and quality control requirements are also unchanged. The key differences lie in the extraction methods and instrumental methods. Briefly, legacy munitions analysis of low concentration waters uses a single cartridge reverse phase SPE procedure, and acetonitrile (ACN) is used for both extraction and elution for aqueous and solid samples[19][20]. An isocratic separation via reversed-phase C-18 column with 50:50 methanol:water mobile phase or a C-8 column with 15:85 isopropanol:water mobile phase is used to separate legacy munitions[19]. While these procedures are sufficient for analysis of legacy munitions, alternative solvents, additional SPE cartridges, and a gradient elution are all required for the combined analysis of legacy and insensitive munitions.
Previously, analysis of legacy and insensitive munitions required multiple analytical techniques, however the methods presented here combine the two munitions categories resulting in an HPLC-UV method and accompanying extraction methods for a variety of common sample matrices. A secondary HPLC-UV method and a HPLC-MS method were also developed as confirmatory methods. The methods discussed in this article were validated extensively by single-blind round robin testing and subsequent statistical treatment as part of ESTCP ER19-5078. Wherever possible, the quality control criteria in the Department of Defense Quality Systems Manual for Environmental Laboratories were adhered to[21]. Analytes included in the methods presented here are found in Table 1.
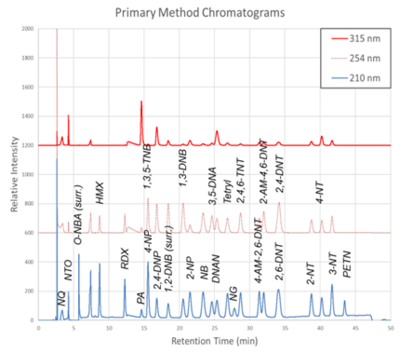
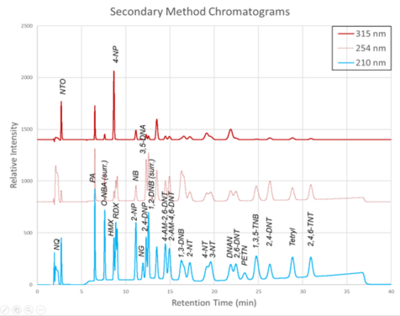
The chromatograms produced by the primary and secondary HPLC-UV methods are shown in Figure 1 and Figure 2, respectively. Chromatograms for each detector wavelength used are shown (315, 254, and 210 nm).
Extraction Methods
High Concentration Waters (> 1 ppm)
Aqueous samples suspected to contain the compounds of interest at concentrations detectable without any extraction or pre-concentration are suitable for analysis by direct injection. The method deviates from USEPA Method 8330B by adding a pH adjustment and use of MeOH rather than ACN for dilution[19]. The pH adjustment is needed to ensure method accuracy for ionic compounds (like NTO or PA) in basic samples. A solution of 1% HCl/MeOH is added to both acidify and dilute the samples to a final acid concentration of 0.5% (vol/vol) and a final solvent ratio of 1:1 MeOH/H2O. The direct injection samples are then ready for analysis.
Low Concentration Waters (< 1 ppm)
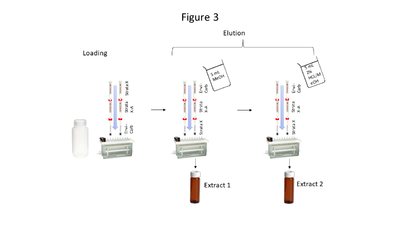
Aqueous samples suspected to contain the compounds of interest at low concentrations require extraction and pre-concentration using solid phase extraction (SPE). The SPE setup described here uses a triple cartridge setup shown in Figure 3. Briefly, the extraction procedure loads analytes of interest onto the cartridges in this order: StrataTM X, StrataTM X-A, and Envi-CarbTM. Then the cartridge order is reversed, and analytes are eluted via a two-step elution, resulting in 2 extracts (which are combined prior to analysis). Five milliliters of MeOH is used for the first elution, while 5 mL of acidified MeOH (2% HCl) is used for the second elution. The particular SPE cartridges used are noncritical so long as cartridge chemistries are comparable to those above.
| Method run time = 48 minutes; Column temperature = 25°C Injection volume = 50 μL; Flow rate = 1.0 mL/min Detector wavelengths = 210, 254, and 310 nm | ||||
| Time (min) |
Reagent Water (%) |
MeOH (%) |
0.1% TFA/Water (%) |
ACN (%) |
|---|---|---|---|---|
| 0.00 | 89 | 3 | 3 | 5 |
| 2.00 | 89 | 3 | 3 | 5 |
| 2.20 | 52 | 40 | 3 | 5 |
| 12.5 | 52 | 40 | 3 | 5 |
| 19.0 | 57 | 35 | 3 | 5 |
| 28.0 | 48 | 44 | 3 | 5 |
| 32.0 | 48 | 44 | 3 | 5 |
| 44.0 | 32 | 60 | 3 | 5 |
| 44.1 | 89 | 3 | 3 | 5 |
| 48.0 | 89 | 3 | 3 | 5 |
Soils
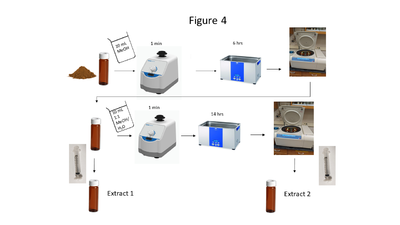
Soil collection, storage, drying and grinding procedures are identical to the USEPA Method 8330B procedures[19]; however, the solvent extraction procedure differs in the number of sonication steps, sample mass and solvent used. A flow chart of the soil extraction procedure is shown in Figure 4. Soil masses of approximately 2 g and a sample to solvent ratio of 1:5 (g/mL) are used for soil extraction. The extraction is carried out in a sonication bath chilled below 20 ⁰C and is a two-part extraction, first extracting in MeOH (6 hours) followed by a second sonication in 1:1 MeOH:H2O solution (14 hours). The extracts are centrifuged, and the supernatant is filtered through a 0.45 μm PTFE disk filter.
The solvent volume should generally be 10 mL but if different soil masses are required, solvent volume should be 5 mL/g. The extraction results in 2 separate extracts (MeOH and MeOH:H2O) that are combined prior to analysis.
Tissues
Tissue matrices are extracted by 18-hour sonication using a ratio of 1 gram of wet tissue per 5 mL of MeOH. This extraction is performed in a sonication bath chilled below 20 ⁰C and the supernatant (MeOH) is filtered through a 0.45 μm PTFE disk filter.
Due to the complexity of tissue matrices, an additional tissue cleanup step, adapted from prior research, can be used to reduce interferences[22][23]. The cleanup procedure uses small scale chromatography columns prepared by loading 5 ¾” borosilicate pipettes with 0.2 g activated silica gel (100–200 mesh). The columns are wetted with 1 mL MeOH, which is allowed to fully elute and then discarded prior to loading with 1 mL of extract and collecting in a new amber vial. After the extract is loaded, a 1 mL aliquot of MeOH followed by a 1 mL aliquot of 2% HCL/MeOH is added. This results in a 3 mL silica treated tissue extract. This extract is vortexed and diluted to a final solvent ratio of 1:1 MeOH/H2O before analysis.
HPLC-UV and HPLC-MS Methods
| Method run time = 43 minutes; Column temperature = 25°C Injection volume = 50 μL; Flow rate = 0.8 mL/min Detector wavelengths = 210, 254, and 310 nm | ||||
| Time (min) |
Reagent Water (%) |
MeOH (%) |
0.1% TFA/Water (%) |
ACN (%) |
|---|---|---|---|---|
| 0.00 | 75 | 10 | 10 | 5 |
| 2.50 | 75 | 10 | 10 | 5 |
| 2.60 | 39 | 46 | 10 | 5 |
| 9.00 | 39 | 46 | 10 | 5 |
| 9.10 | 33.5 | 51.5 | 10 | 5 |
| 15.00 | 35 | 50 | 10 | 5 |
| 15.10 | 43 | 42 | 10 | 5 |
| 33.00 | 30 | 55 | 10 | 5 |
| 33.10 | 75 | 10 | 10 | 5 |
| 43.00 | 75 | 10 | 10 | 5 |
| Parameter | Value |
|---|---|
| Ionization Source | APCI |
| Ionization Mode | Negative |
| Drying Gas Temperature (°C) | 350 |
| Vaporizer Temperature (°C) | 325 |
| Drying Gas Flow (L/min) | 4.0 |
| Nebulizer Pressure (psig) | 40 |
| Corona Current (μA) | 10 |
| Capillary Potential (V) | 1500 |
| Mass Range | 40 – 400 |
| Fragmentor | 100 |
| Gain | 1 |
| Threshold | 0 |
| Step Size | 0.20 |
| Speed (μ/sec) | 743 |
| Peak Width (min) | 0.06 |
| Cycle Time (sec/cycle) | 0.57 |
The Primary HPLC-UV method uses a Phenomenex Synergi 4 µm Hydro-RP column (80Å, 250 x 4.6 mm), or comparable, and is based on both the HPLC method found in USEPA 8330B and previous work[19][22][23]. This separation relies on a reverse phase column and uses a gradient elution, shown in Table 2. Depending on the analyst’s needs and equipment availability, the method has been proven to work with either 0.1% TFA or 0.25% FA (vol/vol) mobile phase. Addition of a guard column like a Phenomenex SecurityGuard AQ C18 pre-column guard cartridge can be optionally used. These optional changes to the method have no impact on the method’s performance.
The Secondary HPLC-UV method uses a Restek Pinnacle II Biphenyl 5 µm (150 x 4.6 mm) or comparable column and is intended as a confirmatory method. Like the Primary method, this method can use an optional guard column and utilizes a gradient elution, shown in Table 3.
For instruments equipped with a mass spectrometer (MS), a secondary MS method is available and was developed alongside the Primary UV method. The method was designed for use with a single quadrupole MS equipped with an atmospheric pressure chemical ionization (APCI) source, such as an Agilent 6120B. A majority of the analytes shown in Table 1 are amenable to this MS method, however nitroglycerine (which is covered extensively in USEPA method 8332) and 2-,3-, and 4-nitrotoluene compounds aren’t compatible with the MS method. MS method parameters are shown in Table 4.
Summary
The extraction methods and instrumental methods in this article build upon prior munitions analytical methods by adding new compounds, combining legacy and insensitive munitions analysis, and expanding usable sample matrices. These methods have been verified through extensive round robin testing and validation, and while the methods are somewhat challenging, they are crucial when simultaneous analysis of both insensitive and legacy munitions is needed.
References
- ^ Risacher, F.F., Schneider, H., Drygiannaki, I., Conder, J., Pautler, B.G., and Jackson, A.W., 2023. A Review of Peeper Passive Sampling Approaches to Measure the Availability of Inorganics in Sediment Porewater. Environmental Pollution, 328, Article 121581. doi: 10.1016/j.envpol.2023.121581 Open Access Manuscript
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Risacher, F.F., Nichols, E., Schneider, H., Lawrence, M., Conder, J., Sweett, A., Pautler, B.G., Jackson, W.A., Rosen, G., 2023b. Best Practices User’s Guide: Standardizing Sediment Porewater Passive Samplers for Inorganic Constituents of Concern, ESTCP ER20-5261. Project Website Report.pdf
- ^ Conder, J.M., Fuchsman, P.C., Grover, M.M., Magar, V.S., Henning, M.H., 2015. Critical review of mercury SQVs for the protection of benthic invertebrates. Environmental Toxicology and Chemistry, 34(1), pp. 6-21. doi: 10.1002/etc.2769 Open Access Article
- ^ 4.0 4.1 Cleveland, D., Brumbaugh, W.G., MacDonald, D.D., 2017. A comparison of four porewater sampling methods for metal mixtures and dissolved organic carbon and the implications for sediment toxicity evaluations. Environmental Toxicology and Chemistry, 36(11), pp. 2906-2915. doi: 10.1002/etc.3884
- ^ 5.0 5.1 Gruzalski, J.G., Markwiese, J.T., Carriker, N.E., Rogers, W.J., Vitale, R.J., Thal, D.I., 2016. Pore Water Collection, Analysis and Evolution: The Need for Standardization. In: Reviews of Environmental Contamination and Toxicology, Vol. 237, pp. 37–51. Springer. doi: 10.1007/978-3-319-23573-8_2
- ^ Peijnenburg, W.J.G.M., Teasdale, P.R., Reible, D., Mondon, J., Bennett, W.W., Campbell, P.G.C., 2014. Passive Sampling Methods for Contaminated Sediments: State of the Science for Metals. Integrated Environmental Assessment and Management, 10(2), pp. 179–196. doi: 10.1002/ieam.1502 Open Access Article
- ^ 7.0 7.1 Teasdale, P.R., Batley, G.E., Apte, S.C., Webster, I.T., 1995. Pore water sampling with sediment peepers. Trends in Analytical Chemistry, 14(6), pp. 250–256. doi: 10.1016/0165-9936(95)91617-2
- ^ Schroeder, H., Duester, L., Fabricius, A.L., Ecker, D., Breitung, V., Ternes, T.A., 2020. Sediment water (interface) mobility of metal(loid)s and nutrients under undisturbed conditions and during resuspension. Journal of Hazardous Materials, 394, Article 122543. doi: 10.1016/j.jhazmat.2020.122543 Open Access Article
- ^ Wise, D.E., 2009. Sampling techniques for sediment pore water in evaluation of reactive capping efficacy. Master of Science Thesis. University of New Hampshire Scholars’ Repository. 178 pages. Website Report.pdf
- ^ 10.0 10.1 Hesslein, R.H., 1976. An in situ sampler for close interval pore water studies. Limnology and Oceanography, 21(6), pp. 912-914. doi: 10.4319/lo.1976.21.6.0912 Open Access Article
- ^ Serbst, J.R., Burgess, R.M., Kuhn, A., Edwards, P.A., Cantwell, M.G., Pelletier, M.C., Berry, W.J., 2003. Precision of dialysis (peeper) sampling of cadmium in marine sediment interstitial water. Archives of Environmental Contamination and Toxicology, 45(3), pp. 297–305. doi: 10.1007/s00244-003-0114-5
- ^ 12.0 12.1 Thomas, B., Arthur, M.A., 2010. Correcting porewater concentration measurements from peepers: Application of a reverse tracer. Limnology and Oceanography: Methods, 8(8), pp. 403–413. doi: 10.4319/lom.2010.8.403 Open Access Article
- ^ Passeport, E., Landis, R., Lacrampe-Couloume, G., Lutz, E.J., Erin Mack, E., West, K., Morgan, S., Lollar, B.S., 2016. Sediment Monitored Natural Recovery Evidenced by Compound Specific Isotope Analysis and High-Resolution Pore Water Sampling. Environmental Science and Technology, 50(22), pp. 12197–12204. doi: 10.1021/acs.est.6b02961
- ^ Carignan, R., St‐Pierre, S., Gachter, R., 1994. Use of diffusion samplers in oligotrophic lake sediments: Effects of free oxygen in sampler material. Limnology and Oceanography, 39(2), pp. 468-474. doi: 10.4319/lo.1994.39.2.0468 Open Access Article
- ^ USEPA, 2017. Laboratory, Field, and Analytical Procedures for Using Passive Sampling in the Evaluation of Contaminated Sediments: User’s Manual. EPA/600/R-16/357. Report.pdf
- ^ Walsh, M.R., Temple, T., Bigl, M.F., Tshabalala, S.F., Mai, N. and Ladyman, M., 2017. Investigation of Energetic Particle Distribution from High‐Order Detonations of Munitions. Propellants, Explosives, Pyrotechnics, 42(8), pp. 932-941. doi: 10.1002/prep.201700089
- ^ Mainiero, C. 2015. Picatinny Employees Recognized for Insensitive Munitions. U.S. Army, Picatinny Arsenal Public Affairs. Open Access Press Release
- ^ Frem, D., 2022. A Review on IMX-101 and IMX-104 Melt-Cast Explosives: Insensitive Formulations for the Next-Generation Munition Systems. Propellants, Explosives, Pyrotechnics, 48(1), e202100312. doi: 10.1002/prep.202100312
- ^ 19.0 19.1 19.2 19.3 19.4 19.5 Cite error: Invalid
<ref>tag; no text was provided for refs named8330B - ^ United States Environmental Protection Agency (USEPA), 2007. EPA Method 3535A (SW-846) Solid-Phase Extraction (SPE), Revision 1. USEPA Website Method 3535A.pdf
- ^ US Department of Defense and US Department of Energy, 2021. Consolidated Quality Systems Manual (QSM) for Environmental Laboratories, Version 5.4. 387 pages. Free Download QSM Version 5.4.pdf
- ^ 22.0 22.1 Russell, A.L., Seiter, J.M., Coleman, J.G., Winstead, B., Bednar, A.J., 2014. Analysis of munitions constituents in IMX formulations by HPLC and HPLC-MS. Talanta, 128, pp. 524–530. doi: 10.1016/j.talanta.2014.02.013
- ^ 23.0 23.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedCrouchEtAl2020