Hydrothermal Alkaline Treatment (HALT)
Hydrothermal alkaline treatment (HALT) is a thermochemical processing technology effective at destroying and defluorinating halogenated organic compounds such as per- and polyfluoroalkyl substances (PFAS). HALT is highly effective at destroying and defluorinating all types of PFAS that have been evaluated. The HALT technology enables end-to-end treatment and destruction of PFAS from a variety of matrices when integrated into a suitable treatment train.
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Ex Situ Water Treatment
- PFAS Sources
- PFAS Transport and Fate
Contributors: Dr. Brian Pinkard, Dr. Timothy Strathmann, and Dr. Shilai Hao
Key Resource(s):
- Hydrothermal Technologies for On-Site Destruction of Site Investigation Wastes Contaminated with Per- and Polyfluoroalkyl Substances (PFAS), Phase I[1]
- Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam[2]
- Degradation and Defluorination of Ultra Short-, Short-, and Long-Chain PFASs in High Total Dissolved Solids Solutions by Hydrothermal Alkaline Treatment ─ Closing the Fluorine Mass Balance[3]
Introduction
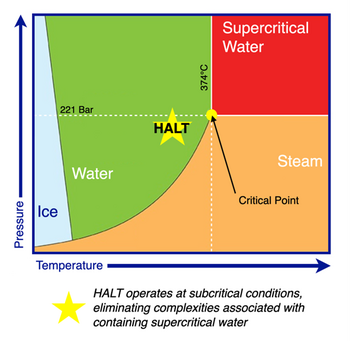
Hydrothermal alkaline treatment (HALT) is a thermochemical processing technology effective at destroying and defluorinating halogenated organic compounds such as per- and polyfluoroalkyl substances (PFAS). HALT is also known as “alkaline hydrolysis,” and is very similar to processing technologies such as hydrothermal liquefaction (HTL) which have been developed and investigated for organic waste-to-energy applications.
HALT processing subjects PFAS in an aqueous solution to high pressure, high temperature, and high pH conditions. The required operating conditions are dependent on the specific target PFAS being destroyed, as perfluorocarboxylic acids (PFCAs) such as trifluoroacetic acid (TFA) can be destroyed under mild conditions (e.g., P ~ 2 MPa, T ~ 200 °C, pH ~ 13)[4], whereas perfluorosulfonic acids (PFSAs) such as perfluorobutanesulfonic acid (PFBS) require more aggressive processing conditions (e.g., P ~ 25 MPa, T ~ 350 °C, pH ~ 14.7) [5] (Figure 1). HALT is capable of facilitating complete “mineralization” of PFAS, defined as the conversion of organic fluorine to dissolved inorganic fluoride. The treatment time for HALT is relatively shorter (<2 hours) compared to most other PFAS destructive technologies. For instance, treatment of two-fold diluted aqueous film-forming foams (AFFFs) using HALT in batch mode achieved nearly complete defluorination in just 30 minutes under conditions of 350 °C and 5 M NaOH[2]. PFCAs can be destroyed with even faster kinetics at milder conditions; for example, >90% destruction and defluorination of trifluoroacetic acid (TFA) was achieved within 40 min at 200 °C[4]. Kinetic rate constants for individual PFAS compounds in HALT environments have been proposed in several studies[4][5]. The fluorine mass balance during HALT processing has also been investigated, showing near-stoichiometric conversion of organic fluorine to inorganic fluoride under optimal conditions[3].
From a practical perspective, HALT is best suited for destroying PFAS in concentrated liquids such as liquid concentrate streams produced as byproducts of other water treatment processes (e.g., regenerable ion exchange, foam fractionation). Previous publications demonstrate that complex sample matrices, including high concentrations of inorganic salts (e.g., 83 g/L chloride) and dissolved organic carbon (e.g., 13 g/L), do not inhibit the degradation rate of PFAS compared to a clean matrix, such as groundwater[6][7]. Moreover, several field demonstrations of HALT have been performed successfully, and the technology is scalable for commercialization.
Reaction Mechanisms and Treatment Efficacy
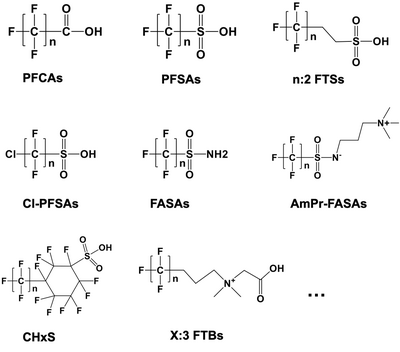
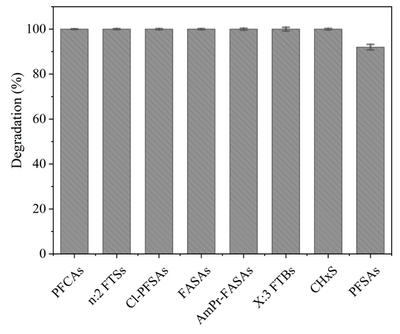
Laboratory scale batch experiments have shown that the full suite of PFAS detected in aqueous film-forming foams (AFFFs) through targeted LC-MS/MS and LC-HRMS suspect screening analysis are degraded and defluorinated by HALT[2]. Figure 2 presents representative classes of PFAS structures among 148 PFAS demonstrating complete degradation during HALT. Figure 3 illustrates the degradation during HALT of representative classes of PFAS detected in an AFFF. The extent of destruction for all PFAS is highly temperature dependent, but results show that some subclasses of PFAS degrade in the absence of alkali amendments (e.g., PFCAs)[4], whereas other subclasses require strong alkali in addition to near-critical reaction temperatures (e.g., PFSAs)[1][2][5]. This is attributed to different mechanisms that initiate the destruction of the individual PFAS subclasses. Degradation of PFCAs is initiated by thermally driven decarboxylation reactions[4], whereas PFSA degradation, in the temperature range of HALT reactors, is proposed to be initiated via attack by the strong nucleophile OH-.[2]
A mechanistic understanding of the HALT process for PFAS destruction needs further evaluation to optimize the process and reduce the consumption of chemicals and energy. While the studies of neat compounds are relatively straightforward, one of the major challenges is to address the effect of co-contaminants and apply the process to real-world operating scenarios. Recent laboratory studies with batch reactors conducted at the Colorado School of Mines (CSM) have extended the application of HALT for the destruction of PFAS in a variety of contaminated matrices, including groundwater and soils[6] and foam fractionation-derived liquid concentrate[7]. Apparent rates for the transformation of individual PFAS have been found to be largely insensitive to the type of media[7], but there is a need to account for any reactions with the media that consume OH· (e.g., OH· reactions with silica-containing soil minerals)[6] Notably, while alkali is not required to degrade PFCAs, it is still necessary to convert the organically bound fluorine to inorganic F-. Austin et. al.[4] showed that TFA, a C1 PFCA, degrades at similar rates in the absence and presence of NaOH, but mineralization to F- and CO32- only occurs when NaOH is added; otherwise fluoroform (CHF3) is the terminal product when no NaOH is added to the reaction solution.
HALT can also be applied to destroy other fluorinated compounds, for example, hydrofluorocarbon (HFC) refrigerants. HFC refrigerants are known to decompose into PFAS such as TFA in the atmosphere and thereby subsequently appear in concerning concentrations in rainwater. By themselves, HFCs are resistant to thermal degradation; however, in the presence of alkali (e.g., NaOH), alkaline hydrolysis can occur at T < 150˚C[4].
State of the Art
Recently, several field demonstrations of pilot-scale HALT systems were performed by commercial HALT provider Aquagga, Inc. These have focused on treating PFAS-rich liquids, including industrial wastewater at a 3M Company facility (April 2024)[8], foam fractionate from a fire training pit in Fairbanks, AK (August 2023) (Figure 4), foam fractionate from groundwater at Beale Air Force Base, CA (May 2024), and AFFF (May 2024). For all field demonstrations, a containerized HALT system was mobilized to the site and operated for up to several weeks. The systems were typically operated at a throughput between 5 and 10 gallons per hour (gph). Since 2019, HALT has progressed from small-scale batch reactors to successful field demonstration of pilot-scale systems. This technology maturation attests to strong technical and regulatory tailwinds. Effort is still needed to demonstrate the technology at full scale and in complex treatment scenarios. Long-term operation of the systems will allow for further optimization of the systems and provide data on the applicability of HALT for the treatment of industrial and environmental PFAS-contaminated waste streams.
Scaled-up HALT systems are typically continuous flow tubular reactor systems, consisting of a single high-temperature, high-pressure fluid path. In commercial HALT systems offered by Aquagga, Inc., chemical dosing for pH adjustment is achieved via an automated chemical dosing and mixing system. The high pH feedstock is then introduced to the high-pressure reactor via a high-pressure metering pump. Pressure is controlled via a back-pressure device downstream of the high-temperature reactor zone. The pressurized reactants are brought to reaction temperatures via a recuperative heat exchanger followed by electric resistive heaters. The reactor vessel contains the reactants at the necessary temperature and pressure and for a sufficient residence time to facilitate the destruction reactions. The product stream is then cooled through a recuperative heat exchanger, before being throttled to ambient pressure through the back-pressure device. Pressure transducers, flow meters, and thermocouples are used to monitor the reactor operations at various points in the system. All reactor components are typically housed within a shipping container, for ease of system transport and to provide secondary chemical containment.
Practical Applications
The ideal use case for HALT is treating PFAS-rich liquid matrices. PFAS concentrations are high enough for HALT to be directly applicable primarily in the cases of AFFF treatment or industrial process water treatment. In the majority of use cases, it is more practical to apply a separation and concentration technology prior to HALT, to reduce the volume of liquid requiring HALT treatment while increasing PFAS concentrations in that liquid. These concentration technologies may include regenerable sorbents, membranes, or foam fractionation, all of which produce a liquid byproduct amenable for HALT.
Destruction of PFAS in Ion Exchange Regeneration Brine
One of the most promising applications of HALT is for treating PFAS-rich ion exchange (IX) regeneration brines, either in site remediation applications (e.g., groundwater treatment[9]) or industrial wastewater treatment applications[3]. IX capture and regeneration involve sorbing PFAS to an IX resin, followed by chemical desorption of PFAS from the resin, typically with a solvent and/or salt wash solution. The IX regeneration technology is commercially mature and available from several vendors.
A treatment train of IX followed by HALT shows promise for several reasons. One reason is that the HALT process is highly compatible with the liquid matrix produced through the IX regeneration. Typically, IX regeneration brine (a.k.a. “still bottoms”) contains high levels of dissolved solids such as sodium chloride, which can cause practical processing challenges with other liquid treatment technologies. However, high levels of TDS do not appear to cause processing challenges with HALT[3]. Another reason is that IX regeneration brines often contain ultra short- and short-chain PFAS, which are amenable to destructive treatment with HALT.
In 2022, commercial HALT provider Aquagga performed a bench study in partnership with the 3M Company, demonstrating PFAS destruction performance for HALT processing of a synthetic IX regeneration brine[3]. Seven treatment conditions were tested, and fluorine mass balance closure was demonstrated for most conditions using a range of analytical techniques. In 2024, Aquagga performed an on-site demonstration in partnership with the 3M Company treating IX regeneration brine produced from active wastewater treatment activities (Figure 5)[8].
Foam Fractionate Treatment
Foam fractionation is a technology that concentrates PFAS in liquids by taking advantage of the hydrophobic/interface-partitioning behavior exhibited by many types of PFAS. Foam fractionation is seeing broad adoption for challenging liquid matrices such as landfill leachate and groundwater. Long-chain PFAS are known to partition to interfaces much more readily than short-chain PFAS, and foam fractionation is correspondingly much more effective at removing long-chain PFAS from liquids. When coupled with HALT, foam fractionation can remove and destroy a high fraction of PFAS from challenging liquid matrices[7].
Destruction of PFAS in AFFF
Legacy AFFF contains high levels of PFAS (typically 0.1 to 6 wt%) in a liquid matrix. Several studies at lab and pilot scales have demonstrated that HALT can destroy PFAS in AFFF with minimal dilution[2]. While the treatment is effective, the wide variety of AFFF formulations make this a challenging application.
Advantages and Drawbacks
Advantages of HALT include:
- Ability to achieve >99% destruction of all PFAS chain lengths and subtypes
- Ability to fully mineralize or defluorinate PFAS to dissolved inorganic fluoride as an end product
- Commercial systems are compact and simple to operate
- Commercial systems do not have an air emission point
- Ability to treat wastes with high TDS
- Ability to treat wastes with high TOC
- Low overall energy usage (<0.9 kWh/gal-treated)
Drawbacks or challenges associated with HALT include:
- Not well-suited for directly processing solid materials or slurries
- Treated effluent brine contains high TDS and must be managed accordingly
- Hard minerals (e.g., Ca2+) may precipitate and require periodic cleaning
- The use of strong bases and conjugate acids require safe chemical handling practices external to the HALT system and appropriate operator precautions
- High-pressure, high-temperature, and high-pH operating conditions are harsh and corrosive on processing equipment, and appropriate material selection, metallurgy, and corrosion control methods must be applied to ensure reactor vessel reliability
References
- ^ 1.0 1.1 Strathmann, T.J., Higgins, C., Deeb, R., 2020. Final Report: Hydrothermal Technologies for On-Site Destruction of Site Investigation Wastes Contaminated with Per- and Polyfluoroalkyl Substances (PFAS), Phase I. Strategic Environmental Research and Development Program (SERDP) Project number ER18-1501. Final Report pdf Project Website
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Hao, S., Choi, Y.J., Wu, B., Higgins, C.P., Deeb, R., Strathmann, T.J., 2021. Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam. Environmental Science and Technology, 55(5), pp. 3283-3295. doi: 10.1021/acs.est.0c06906
- ^ 3.0 3.1 3.2 3.3 3.4 Pinkard, B., Smith, S.M., Vorarath, P., Smrz, T., Schmick, S., Dressel, L., Bryan, C., Czerski, M., de Marne, A., Halevi, A., Thomsen, C., Woodruff, C., 2024. Degradation and Defluorination of Ultra Short-, Short-, and Long-Chain PFASs in High Total Dissolved Solids Solutions by Hydrothermal Alkaline Treatment─Closing the Fluorine Mass Balance. ACS ES&T Engineering, 4(11), pp. 2810-2818. doi: 10.1021/acsestengg.4c00378 Report pdf
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Austin, C., Purohit, A., Thomsen, C., Pinkard, B.R., Strathmann, T.J., Novosselov, I.V., 2024. Hydrothermal Destruction and Defluorination of Trifluoroacetic Acid (TFA). Environmental Science and Technology, 58(18), pp. 8076-8085. doi: 10.1021/acs.est.3c09404
- ^ 5.0 5.1 Wu, B., Hao, S., Choi, Y.J., Higgins, C.P., Deeb, R., Strathmann, T.J., 2019. Rapid Destruction and Defluorination of Perfluorooctanesulfonate by Alkaline Hydrothermal Reaction. Environmental Science and Technology Letters, 6(10), pp. 630-636. doi: 10.1021/acs.estlett.9b00506
- ^ 6.0 6.1 6.2 Hao, S., Choi, Y.J,. Deeb, R.A., Strathmann, T.J., Higgins, C.P., 2022. Application of Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Contaminated Groundwater and Soil. Environmental Science and Technology, 56(10), pp. 6647-6657. doi: 10.1021/acs.est.2c00654
- ^ 7.0 7.1 7.2 7.3 Hao, S., Reardon, P.N., Choi, Y.J., Zhang, C., Sanchez, J.M., Higgins, C.P., Strathmann, T.J., 2023. Hydrothermal Alkaline Treatment (HALT) of Foam Fractionation Concentrate Derived from PFAS-Contaminated Groundwater. Environmental Science and Technology 57(44), pp. 17154-17165. doi: 10.1021/acs.est.3c05140
- ^ 8.0 8.1 Pinkard, B.R., Smith, S.M., Bryan, C., 2024. PFAS Degradation and Defluorination of High TDS Wastewater via Continuous Hydrothermal Alkaline Treatment (HALT). In: (Proceedings of the) 85th Annual International Water Conference (IWC 2024), Volume 1, pp. 359-374. Engineers Society of Western Pennsylvania. ISBN: 979-8-3313-1299-2
- ^ Pinkard, B.R., 2024. Hydrothermal Alkaline Treatment for a Closed-Loop, On-Site PFAS Treatment Solution. Project Number ER23-8400, Environmental Security Technology Certification Program (ESTCP). Project Website

