Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
Environmental releases of perfluoroalkyl and polyfluoroalkyl substances (PFAS) including perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS)have occurred at manufacturing facilities and in areas where aqueous film-forming foam (AFFF) was used to extinguish hydrocarbon fires. PFAS are suspected to cause adverse human health effects. They are highly stable in the environment and are typically removed from water supplies using granular activated carbon. There is a need for in situ treatment technologies and ex situ treatment methods that are more cost-effective.
Related Article(s):
CONTRIBUTOR(S): Dr. Rula Deeb, Dr. Jennifer Field, Elisabeth Hawley, and Dr. Christopher Higgins
Key Resource(s):
Introduction
Awareness of PFAS in the environment first emerged in the late 1990s following developments in analytical methods to detect ionized substances. Legal actions were taken against PFAS product manufacturing facilities in the West Virginia/Ohio River Valley[2]. In 2000, the sole U.S. manufacturer of PFOS agreed to voluntarily discontinue production[3]. The U.S. Environmental Protection Agency (EPA) issued provisional drinking water health advisories for PFOA and PFOS in 2009 and replaced these with health advisories in 2016[4]. Over the past five years, state regulators have required several former Air Force and Navy fire-fighter training areas to conduct site investigations for PFAS. SERDP/ESTCP research programs began funding related research in 2011 because they recognized the potential impact of this issue for the Department of Defense.
Physical and Chemical Properties
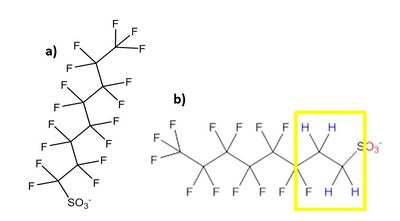
Although the environmental remediation industry initially used the term “perfluorinated compounds” (or PFCs), the more specific terminology of PFAS was recommended for consistent communication within the global scientific, regulatory, and industrial communities[5]. PFAS are fluorinated substances with a carbon chain structure. In perfluoroalkyl substances, each carbon atom in the chain is fully saturated with fluorine (carbon-fluorine bonds only), whereas the carbon chain in polyfluoroalkyl substances is mostly saturated with fluorine (carbon-fluorine bonds), but also contains carbon-hydrogen bonds (Fig. 1).
The most studied PFAS are PFOA and PFOS. Both have a hydrophobic carbon chain structure of eight carbons that are fully saturated with fluorine atoms (i.e., perfluoroalkyl substances) and a hydrophilic polar functional group. They are therefore “ amphiphilic” and associate with water and oils. This property made them useful ingredients in fire-fighting foams and other surfactant applications. In most groundwater environments, PFOS and PFOA are water-soluble anions. Their surfactant properties complicate the prediction of their physiochemical properties, such as partitioning coefficients. The strength of the carbon-fluorine bonds in PFAS creates extremely high chemical and thermal stabilities. Relevant properties of PFOS and PFOA are summarized below (Table 1[1]).

Environmental Concern
Perfluorinated substances are very stable, do not biodegrade, and are found throughout the environment globally. In contrast, the presence of carbon-hydrogen groups in polyfluoroalkyl substances makes these compounds easier to partially degrade, forming shorter-chain perfluoroalkyl compounds. Trace amounts of perfluorinated substances have been detected at remote locations like the Arctic, far from potential point sources[6]. Other studies have shown that long-chain perfluorinated substances bioaccumulate and biomagnify in wildlife[7]. Because of this, higher trophic wildlife including fish and birds can be particularly susceptible[8]. The Dutch National Institute for Public Health and the Environment calculated a maximum permissible concentration for PFOS of 0.65 nanograms per liter (ng/L) for fresh water, based on human consumption of fish[1].
PFAS typically associate with the liver, proteins, and the blood stream. In humans, they have a half-life in the range of 2 to 9 years[1]. Toxicological studies of PFOA indicate potential developmental or reproductive effects[1]. Both PFOA and PFOS are suspected carcinogens, but their carcinogenicity remains to be classified by the U.S. EPA[1]. The International Agency for Research on Cancer (IARC) has classified PFOA as a Group 2B carcinogen, i.e., possibly carcinogenic to humans[9][10]. The U.S. EPA published draft reference doses of 30 ng/kg*day PFOS and 20 ng/kg*day PFOA (based on non-cancer hazard). For site remediation, drinking water ingestion, fish consumption, dermal contact with water, and (accidental) ingestion or contact with contaminated soil are the exposure pathways of concern.
Uses and Potential Sources to the Environment
Due to their unique properties, many PFAS function as surfactants or components of surface coatings. They are stain-resistant, heat-resistant, and are useful for coating surfaces that are in contact with acids or bases[11][1]. Thus, they are used widely by a number of industries, including carpet, textile and leather production, chromium plating, photography, photolithography, semi-conductor manufacturing, coating additives, cleaning products, and insecticides[1]. PFAS are also found in a variety of consumer products including food paper and packaging, furnishings, waterproof clothing, and cosmetics[12]. The presence of PFASs in consumer products has created an urban background concentration in stormwater, wastewater treatment plant influent[13], and landfill leachate[14].
One of the most widely known sources of PFAS is AFFF, which was used in large quantities in the environment on fires, at fire-fighting training areas, during the activation of fire suppression systems in airplane hangars and other buildings, and accidentally through AFFF storage, transport, and day-to-day handling. AFFF was routinely used at military sites, airports, and refineries. Formulations are proprietary and the composition of AFFF varies with the manufacturer. However, AFFF typically consists of water (60-93%), solvents such as butyl carbitol (3-25%), hydrocarbon surfactants (1-12%), one or more PFASs, and other compounds (e.g., corrosion inhibitors, electrolytes[15]). PFAS signatures of a variety of different AFFF formulations can assist in forensic identification of PFAS sources[16][17].
Regulation
Final regulations have not yet been promulgated for PFAS; current criteria for PFAS are typically in the form of guidance or advisory levels (Table 2). The U.S. EPA recently developed Drinking Water Health Advisory levels for PFOA and PFOS, replacing previously published provisional values. Several states including Minnesota, Maine and New Jersey, have published screening values or interim criteria for one or more PFAS including PFOS, PFOA, perfluorobutanesulfonic acid (PFBS), perfluorobutanoic acid (PFBA) and perfluorononanoic acid (PFNA) (Table 2). Drinking water, groundwater, and soil criteria in the European Union was recently published in a summary report[18].
Other regulatory actions have restricted the use and production of PFAS. PFOS was added to list of chemicals under the Stockholm Convention on persistent organic pollutants in 2009. Nearly all use of PFOS is therefore banned in Europe, with some exemptions. Substances or mixtures may not contain PFOS above 0.001% by weight (EU 757/2010). In the U.S., because PFOS manufacturing was voluntarily phased out in 2002, AFFF containing PFOS is no longer manufactured. The U.S. military and others still have large quantities of stockpiled AFFF containing PFOS, although its use is discouraged.
| REGULATORY AGENCY | DESCRIPTION | PFOS | PFOA | PFBS | PFBA | PFNA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRINKING WATER (µg/L) | |||||||||||
| U.S. EPA | Drinking Water Health Advisories | 0.07 | 0.07 | ||||||||
| Health Canada | Drinking Water Screening Values | 0.6 | 0.2 | 15 | 30 | 0.2 | |||||
| Maine Department of Environmental Protection | Maximum Exposure Guideline | 0.1 | |||||||||
| Michigan Department of Environmental Quality | Drinking Water Surface Water Quality Value | 0.011 | 0.42 | ||||||||
| New Jersey Department of Environmental Protection | Preliminary Health-Based Guidance Value | 0.04 | |||||||||
| New Jersey Department of Environmental Protection | Development of MCL Recommendations for PFOA and PFOS are Currently in Progress | 0.04 | |||||||||
| New Jersey Department of Environmental Protection | Health-Based Maximum Contaminant Level (MCL) Recommendation | 0.013 | |||||||||
| Vermont Department of Health | Drinking Water Health Advisory Level | 0.02 | |||||||||
| GROUNDWATER (µg/L) | |||||||||||
| Minnesota Department of Health | Health Risk Limit for Groundwater | 0.3 | 0.3 | 7 | 7 | ||||||
| Illinois Environmental Protection Agency | Provisional Groundwater Remediation Objectives, Class I Groundwater | 0.2 | 0.4 | ||||||||
| Illinois Environmental Protection Agency | Provisional Groundwater Remediation Objectives, Class II Groundwater | 0.2 | 0.2 | ||||||||
| North Carolina Department of Environmental Quality | Interim Maximum Allowable Concentration | 1.0 | |||||||||
| New Jersey Department of Environmental Protection | Interim Specific Ground Water Quality Criterion | 0.01 | |||||||||
| Maine Department of Environmental Protection | Remedial Action Guidelines for Residential Groundwater | 0.06 | 0.1 | ||||||||
| Michigan Department of Environmental Quality | Groundwater Residential Generic Cleanup Criteria and Screening Levels | 0.12 | 0.089 | ||||||||
| Michigan Department of Environmental Quality | Groundwater Nonresidential Generic Cleanup Criteria and Screening Levels | 0.5 | 0.28 | ||||||||
| Texas Commission on Environmental Quality Texas Risk Reduction Program | Protective Concentration Levels for 16 PFAS for Several Different Exposure Scenarios (Groundwater) | ||||||||||
| Alaska Department of Environmental Conservation | Cleanup Levels | 0.4 | 0.4 | ||||||||
| SOIL (mg/kg) | |||||||||||
| U.S. EPA Region 4 | Residential Soil Screening Level | 6 | 16 | ||||||||
| Minnesota Pollution Control Agency | Industrial Soil Reference Value (.xlsx) | 14 | 13 | 500 | |||||||
| Minnesota Pollution Control Agency | Residential Soil Reference Value(.xlsx) | 2.1 | 2.1 | 77 | |||||||
| Minnesota Pollution Control Agency | Recreational Soil Reference Value(.xlsx) | 2.6 | 2.5 | 95 | |||||||
| Maine Department of Environmental Protection | Remedial Action Guidelines for different exposure scenarios | 11-82 | |||||||||
| Texas Commission on Environmental Quality Texas Risk Reduction Program | Protective Concentration Levels for 16 PFAS for Several Different Exposure Scenarios (Soil) | ||||||||||
| Alaska Department of Environmental Conservation | Cleanup Level, Arctic Zone | 2.2 | 2.2 | ||||||||
| Alaska Department of Environmental Conservation | Cleanup Level, Under 40' Zone | 1.6 | 1.6 | ||||||||
| Alaska Department of Environmental Conservation | Cleanup Level, Over 40' Zone | 1.3 | 1.3 | ||||||||
| Alaska Department of Environmental Conservation | Cleanup Level, Migration to Groundwater (MTGW) | 0.0030 | 0.0017 | ||||||||
| Table 2. Summary of PFAS Regulatory Criteria. Regulatory criteria for PFAS are still evolving relatively quickly. Please check the hyperlinked reference to confirm that the regulatory criteria listed in the table are up to date before using this information. Some states have PFAS regulatory values for groundwater as a result of consent agreements (e.g., both West Virginia and Ohio signed a consent agreement with DuPont listing 0.4 µg/L as a precautionary site-specific action level for PFOA). Other states (e.g., Delaware, New Hampshire, New York) have adopted U.S. EPA provisional health advisory levels for PFOS and PFOA in several water systems. Pennsylvania has investigated PFOS contamination associated with two contaminated wells identified through EPA Unregulated Contaminant Monitoring Rule program. Alabama has also addressed PFAS contamination on a site-specific basis. Alaska has conducted sampling and monitoring for PFAS at multiple sites. | |||||||||||
Sampling and Analytical Methods
Because PFAS are present in several common consumer items, care should be taken during sampling to eliminate contact with other potential sources of PFAS. Most standard operating procedures and work plans advise avoiding the use of polytetrafluoroethylene-based (e.g., Teflon) components including tubing and lined sample bottle caps. Some also instruct samplers not to wear waterproof jackets or other outerwear with a waterproof coating, and to avoid handling packaged foods that may contain fluorotelomer-based chemicals to increase non-stick properties. Due to the affinity of PFAS for the air-water interface and the wettability of glass, sample bottles are typically polypropylene or high-density polyethylene.
Most commercial laboratories use a modified version of U.S. EPA Method 537 for the analysis of PFAS in drinking water. This method consists of solid phase extraction and liquid chromatography with tandem mass spectrometry. Analytes include PFOS, PFOA, and typically 12 other PFAS (mostly perfluorocarboxylic acids and perfluorosulfonic acids) of varying carbon chain length. Specialty laboratories have modified this analytical method for matrices other than drinking water, to better recover shorter-chain compounds, or achieve lower detection limits.
Commercial laboratories that can quantify an even broader suite of PFAS (e.g., those known to be present in AFFF formulations and degrade to form PFOA and PFOS) are rare. An analytical method to detect several families of PFAS precursors[19]. There is also the Total Oxidizable Precursor (TOP) assay, a bulk measurement of precursors that can be oxidized to perfluorocarboxylates[20]. Other approaches to quantify the total amount of organic fluorine in water samples include particle induced gamma-ray emission (PIGE) and absorbable organic fluorine (AOF)[21].
The cost-effectiveness of high-resolution site characterization methods for PFAS is currently limited due to the lack of a reliable analytical method that can be used in the field as a screening method. Several research groups have attempted to design a field-ready mobile analytical method. For example, United Science LLC is developing ion selective electrodes to measure PFOS at ng/L levels[22]. Geosyntec Consultants and Eurofins Eaton Analytical are developing a mobile field unit for screening PFOS and other PFAS to ng/L levels[23].
Fate and Transport
The following summarize some key concepts for PFAS fate and transport:
- Sorption: Both PFOA and PFOS are anions at typical environmental pH values, but still exhibit strong interactions with solid-phase organic carbon. For this reason, the foc-Koc method for predicting sorption is generally appropriate[24], though this has not been confirmed for all PFAS. Interactions with mineral phases, particularly ferric oxide materials, may be important in low f foc materials[25][26]. At present, empirical site-specific sorption estimates are recommended to accurately predict PFAS mobility[25].
- Biotransformation: PFOS, PFOA, and analogous compounds of varying chain lengths are persistent in the environment and do not readily biodegrade. Polyfluorinated forms partially degrade in the environment[27][28], particularly if conditions (e.g., dissolved oxygen concentrations, pH) have been altered to treat co-contaminants[29]. However, degradation products are often more recalcitrant – degradable polyfluorinated forms are precursors for PFOA, PFOS and their homologs. In contrast, fungal degradation has been shown to result in lower production of perfluorocarboxylic acids[27].
- Other effects of microbes: Some microbes, in the presence of PFOA, aggregate and produce extracellular polymeric substances[30]. Microbes also facilitate PFAS leaching under methanogenic conditions common at municipal solid waste landfills[31]. Depending on the conditions, microbial activity may therefore enhance the mobility of compounds like PFOS and PFOA or hypothetically have the opposite effect by increasing sorption.
- Effect of co-contaminants and co-contaminant remediation strategies: Interactions between PFAS and non-aqueous phase liquids can retard PFAS migration[32]. TCE dechlorination can be inhibited by PFAS[33] and that inhibition depends both on PFAS structure and[34]. PFAS precursors degraded to form PFOA and other PFAS at a former fire-fighting training area at Ellsworth Air Force Base, where several remediation methods, including soil vapor extraction, groundwater pump and treat, bioventing, and oxygen infusion were used to treat co-contaminants[29].
Soil and Groundwater Remediation
Due to the chemical and thermal stability of PFAS and the complexity of PFAS mixtures, soil and groundwater remediation is challenging and costly. Research is still ongoing to develop effective remedial strategies.
For soil, it is common to evaluate several management options: 1) treatment and/or direct on-site reuse, 2) temporary on-site storage, and 3) off-site disposal to a soil processing or treatment facility, licensed landfill, or incinerator. Soil treatment products are commercially available to stabilize PFAS and decrease leaching. Criteria for stabilizing or treating soils prior to landfill disposal are highly site specific. Other technologies that have been considered for removing PFAS from soil include soil washing and incineration.
For groundwater, management options include the following: 1) in situ treatment, 2) ex situ treatment and/or reuse, aquifer reinjection, or discharge to surface water, stormwater, or sewer, 3) temporary on-site storage, and 4) off-site disposal to a hazardous waste treatment and disposal facility. The most common remediation approach is to use pump-and-treat with granular activated carbon followed by off-site incineration of the spent activated carbon. This technology has been used for years at full scale[35]. However, granular activated carbon has a relatively low capacity for PFAS particularly when shorter-chain compounds are present. Sorption capacity improvement tests have been conducted on various forms of granular and powdered activated carbon, ion exchange, and other sorbent materials and mixtures of clay, powdered activated carbon, and other sorbents[36].
Other methods for ex situ PFAS removal include high-pressure membrane treatment using nanofiltration or reverse osmosis. Membrane technologies at full-scale municipal water treatment facilities have effectively removed PFAS[35]. For typical environmental remediation applications, however, membrane treatment has a higher cost than activated carbon and effectiveness can be impaired by other groundwater contaminants[37]. Neutral PFAS, such as the perfluoroalkyl sulfonamides, may not be sufficiently removed[38].
PFAS Treatment Research
PFAS treatment research includes the following topics:
- PFAS Sequestration: Sorbents are being investigated with the long-term goal of using them in an in situ barrier as a low-cost, long-term treatment solution, combined with a method for periodically regenerating or renewing the emplaced sorbent material and treating waste streams on site using ex-situ chemical oxidation (ESTCP project 2423[39]). SERDP/ESTCP has also funded research (ESTCP project ER-2425) to test in situ injection of chemical coagulants (e.g., polyaluminum chloride, cationic polymers) to aid with sorption[40].
- Proof-of-Concept for Biological Treatment: Fungi have been used successfully to degrade PFAS under laboratory conditions[27][41], but are more difficult to maintain in situ. New work (ESTCP project ER-2422) is focused on the viability of packaging the PFAS-degrading enzymes from wood-rotting fungi into “vaults” (naturally-occurring particles found in a wide variety of microorganisms) and using bioaugmentation for in situ degradation[42][43].
- Advanced Oxidation Processes: Advanced oxidation processes for PFAS include electrochemical oxidation, photolysis, and photocatalysis[43]. Electrocatalytic and catalytic approaches using Ti/RuO2 and other mixed metal oxide anodes have been used to oxidize PFAS in the laboratory under a range of conditions (ESTCP project 2424[44]).
- In Situ Chemical Reduction: Methods being investigated include the use of zero-valent metals/bimetals (Pd/Fe, Mg, Pd/Mg) with clay interlayers and co-solvent assisted Vitamin B12 defluorination. One ongoing project (SERDP project ER-2426) focuses on PFOS, which is recalcitrant to many oxidation processes[45]. Reductive technologies could be used as a first step in remediating PFOS and other PFAS.
Summary
PFAS are present in the environment and pose several challenges. Perfluoroalkyl substances are highly stable and can biomagnify in wildlife. Health-based advisory levels are low, i.e., ng/L concentrations in groundwater and drinking water. As awareness of PFAS grows and regulatory criteria evolve, site managers are conducting site investigation, improving analytical techniques, and designing and operating remediation systems. SERDP/ESTCP-funded research aims to demonstrate effective treatment technologies for PFAS and improve technology cost-effectiveness.
References
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 U.S. Environmental Protection Agency, 2014. Emerging contaminants – perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Fact sheet. March Fact Sheet
- ^ Rich, N., 2016. The lawyer who became DuPont’s worst nightmare. The New York Times Magazine.
- ^ United States Environmental Protection Agency (U.S. EPA), 2000. EPA and 3M announce phase out of PFOS. News release dated Tuesday May 16. U.S. EPA PFOS Phase Out Announcement
- ^ United States Environmental Protection Agency (U.S. EPA), 2016. Drinking water health advisories for PFOA and PFOS. U.S. EPA Water Health Advisories - PFOA and PFOS
- ^ Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A. and van Leeuwen, S.P., 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environmental Assessment and Management, 7(4), 513-541. doi: 10.1002/ieam.258
- ^ Young, C.J., Furdui, V.I., Franklin, J., Koerner, R.M., Muir, D.C. and Mabury, S.A., 2007. Perfluorinated acids in arctic snow: new evidence for atmospheric formation. Environmental Science & Technology, 41(10), 3455-3461. doi: 10.1021/es0626234
- ^ Conder, J.M., Hoke, R.A., Wolf, W.D., Russell, M.H. and Buck, R.C., 2008. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environmental Science & Technology, 42(4), 995-1003. doi: 10.1021/es070895g
- ^ Sinclair, E., Mayack, D.T., Roblee, K., Yamashita, N. and Kannan, K., 2006. Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Archives of Environmental Contamination and Toxicology, 50(3), pp.398-410. doi: 10.1007/s00244-005-1188-z
- ^ Benbrahim-Tallaa, L., Lauby-Secretan, B. Loomis, D., Guyton, K.Z., Grosse, Y., Bouvard, F. El Ghissassi, V., Guha, N., Mattock, H., Straif, K., 2014. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. The Lancet Oncology, 15 (9), 924-925. doi: 10.1016/S1470-2045(14)70316-X
- ^ International Agency for Research on Cancer (IARC), 2016. Monographs on the evaluation of carcinogenic risks to humans. Lists of Classifications, Volumes 1 to 116. List of Classifications.pdf
- ^ Krafft, M.P. and Riess, J.G., 2015. Selected physicochemical aspects of poly-and perfluoroalkylated substances relevant to performance, environment and sustainability - Part one. Chemosphere, 129, 4-19. doi: 10.1016/j.chemosphere.2014.08.039
- ^ Birnbaum, L.S. and Grandjean, P., 2015. Alternatives to PFAS: Perspectives on the Science. Environmental Health Perspectives, 123(5), A104-A105. doi: 10.1289/ehp.1509944
- ^ Houtz, E.F., 2013. Oxidative measurement of perfluoroalkyl acid precursors: Implications for urban runoff management and remediation of AFFF-contaminated groundwater and soil. Ph.D. Dissertation. Available online at http://escholarship.org/uc/item/4jq0v5qp
- ^ Lang, J.R., Allred, B.M., Peaslee, G.F., Field, J.A. and Barlaz, M.A., 2016. Release of Per-and Polyfluoroalkyl Substances (PFAS) from Carpet and Clothing in Model Anaerobic Landfill Reactors. Environmental Science & Technology, 50(10), 5024-5032. doi: 10.1021/acs.est.5b06237
- ^ Conder, J., Deeb, R.A., Field, J.A. and Higgins, C.P., 2016. GRACast: Frequently asked questions on Per- and Polyfluoroalkyl Substances (PFAS). Presented on July 6. FAQs
- ^ Backe, W.J., Day, T.C. and Field, J.A., 2013. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from US military bases by nonaqueous large-volume injection HPLC-MS/MS. Environmental Science & Technology, 47(10), 5226-5234. doi: 10.1021/es3034999
- ^ Place, B.J. and Field, J.A., 2012. Identification of novel fluorochemicals in aqueous film-forming foams used by the U.S. military. Environmental Science & Technology, 46(13), 7120-7127. doi: 10.1021/es301465n
- ^ Concawe, 2016. Environmental fate and effects of poly- and perfluoroalkyl substances (PFAS). Report no. 8/16. Report pdf
- ^ TerMaath, S., J. Field and C. Higgins, 2016. Per- and polyfluoroalkyl substances (PFAS): Analytical and characterization frontiers. Webinar Series
- ^ Houtz, E.F., Higgins, C.P., Field, J.A. and Sedlak, D.L., 2013. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental Science & Technology, 47(15), 8187-8195. doi: 10.1021/es4018877
- ^ Willach, S., Brauch, H.J. and Lange, F.T., 2016. Contribution of selected perfluoroalkyl and polyfluoroalkyl substances to the adsorbable organically bound fluorine in German rivers and in a highly contaminated groundwater. Chemosphere, 145, 342-350. doi:10.1016/j.chemosphere.2015.11.113
- ^ U.S. Environmental Protection Agency, 2015. Final report: field deployable PFCs sensors for contaminated soil screening. EPA contract number EPD14012. Report pdf
- ^ Deeb, R., Chambon, J., Haghani, A., and Eaton, A., 2016. Development and testing of an analytical method for real time measurement of polyfluoroalkyl and perfluoroalkyl substances (PFAS). Presented at the Battelle Chlorinated Conference, Palm Springs, CA.
- ^ Higgins, C.P., and Luthy, R.G., 2006. Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology, 40(23), 7251-7256. doi: 10.1021/es061000n
- ^ 25.0 25.1 Ferrey, M.L., Wilson, J.T., Adair, C., Su, C., Fine, D.D., Liu, X. and Washington, J.W., 2012. Behavior and fate of PFOA and PFOS in sandy aquifer sediment. Groundwater Monitoring & Remediation, 32(4), 63-71. doi: 10.1111/j.1745-6592.2012.01395.x
- ^ Johnson, R.L., Anschutz, A.J., Smolen, J.M., Simcik, M.F. and Penn, R.L., 2007. The adsorption of perfluorooctane sulfonate onto sand, clay, and iron oxide surfaces. Journal of Chemical & Engineering Data, 52(4), 1165-1170. doi: 10.1021/je060285g
- ^ 27.0 27.1 27.2 Tseng, N., Wang, N., Szostek, B. and Mahendra, S., 2014. Biotransformation of 6: 2 fluorotelomer alcohol (6: 2 FTOH) by a wood-rotting fungus. Environmental Science & Technology, 48(7), 4012-4020. doi:10.1021/es4057483
- ^ Harding-Marjanovic, K.C., Houtz, E.F., Yi, S., Field, J.A., Sedlak, D.L. and Alvarez-Cohen, L., 2015. Aerobic biotransformation of fluorotelomer thioether amido sulfonate (Lodyne) in AFFF-amended microcosms. Environmental Science & Technology, 49(13), pp.7666-7674. doi: 10.1021/acs.est.5b01219
- ^ 29.0 29.1 McGuire, M.E., Schaefer, C., Richards, T., Backe, W.J., Field, J.A., Houtz, E., Sedlak, D.L., Guelfo, J.L., Wunsch, A. and Higgins, C.P., 2014. Evidence of remediation-induced alteration of subsurface poly-and perfluoroalkyl substance distribution at a former firefighter training area. Environmental Science & Technology, 48(12), 6644-6652. doi: 10.1021/es5006187
- ^ Weathers, T.S., Higgins, C.P. and Sharp, J.O., 2015. Enhanced biofilm production by a toluene-degrading rhodococcus observed after exposure to perfluoroalkyl acids. Environmental Science & Technology, 49(9), 5458-5466. doi: 10.1021/es5060034
- ^ Allred, B.M., Lang, J.R., Barlaz, M.A. and Field, J.A., 2015. Physical and biological release of poly-and perfluoroalkyl substances (PFAS) from municipal solid waste in anaerobic model landfill reactors. Environmental Science & Technology, 49(13), 7648-7656. doi: 10.1021/acs.est.5b01040
- ^ Guelfo, J. 2013. Subsurface fate and transport of poly- and perfluoroalkyl substances. Doctor of Philosophy Thesis, Colorado School of Mines. Thesis
- ^ Weathers, T.S., Harding-Marjanovic, K., Higgins, C.P., Alvarez-Cohen, L. and Sharp, J.O., 2015. Perfluoroalkyl acids inhibit reductive dechlorination of trichloroethene by repressing dehalococcoides. Environmental Science & Technology, 50(1), 240-248. doi: 10.1021/acs.est.5b04854
- ^ Harding-Marjanovic, K.C., Yi, S., Weathers, T.S., Sharp, J.O., Sedlak, D.L. and Alvarez-Cohen, L., 2016. Effects of Aqueous Film-Forming Foams (AFFFs) on Trichloroethene (TCE) Dechlorination by a Dehalococcoides mccartyi-Containing Microbial Community. Environmental Science & Technology, 50(7), 3352-3361. doi: 10.1021/acs.est.5b04773
- ^ 35.0 35.1 Appleman, T.D., Higgins, C.P., Quinones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C. and Dickenson, E.R., 2014. Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems. Water Research, 51, 246-255. doi: 10.1016/j.watres.2013.10.067
- ^ Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J. and Yu, G., 2014. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents-A review. Journal of Hazardous Materials, 274, 443-454. doi:10.1016/j.jhazmat.2014.04.038
- ^ Department of the Navy (DON). 2015. Interim perfluorinated compounds (PFCs) guidance/frequently asked questions. FAQs
- ^ Steinle-Darling, E. and Reinhard, M., 2008. Nanofiltration for trace organic contaminant removal: structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environmental Science & Technology, 42 (14), 5292–5297. doi: 10.1021/es703207s
- ^ Crimi, M. 2014. In situ treatment train for remediation of perfluoroalkyl contaminated groundwater: In situ chemical oxidation of sorbed contaminants (ISCO-SC), ER-2423. ER-2423
- ^ Simcik, M. (2014). Development of a novel approach for in situ remediation of PFC contaminated groundwater systems, ER-2425. ER-2425
- ^ Qingguo, J. H., 2013. Remediation of perfluoroalkyl contaminated aquifers using an In-situ two-layer barrier: laboratory batch and column study. ER-2127
- ^ Mahendra, S., 2014. Bioaugmentation with vaults: novel in situ remediation strategy for transformation of perfluoroalkyl compounds, SERDP, ER-2422. ER-2422
- ^ 43.0 43.1 Merino, N., Qu, Y., Deeb, R.A., Hawley, E.L., Hoffman, M.R and Mahendra, S., 2016. Degradation and removal methods for perfluoroalkyl and polyfluoroalkyl substances (PFAS) in water. Environmental Engineering Science, 33(9), 615-649. doi:10.1089/ees.2016.0233
- ^ Schaefer, C., 2014. Investigating electrocatalytic and catalytic approaches for in situ treatment of perfluoroalkyl contaminants in groundwater, ER-2424. ER-2424
- ^ Lee, L., 2014. Quantification of in situ chemical reductive defluorination (ISCRD) of perfluoroalkyl acids in groundwater impacted by AFFFs, ER-2426. ER-2426
See Also
Relevant Ongoing SERDP/ESTCP Projects:
- In situ treatment train for remediation of perfluoroalkyl contaminated groundwater: In situ chemical oxidation of sorbed contaminants (ISCO-SC). SERDP/ESTCP Project ER-2423
- Quantification of In Situ Chemical Reductive Defluorination (ISCRD) of perfluoroalkyl acids in groundwater impacted by AFFFs. SERDP/ESTCP Project ER-2426
- Bioaugmentation with vaults: Novel In Situ Remediation Strategy for Transformation of Perfluoroalkyl Compounds. SERDP/ESTCP Project ER-2422
- Investigating Electrocatalytic and Catalytic Approaches for In Situ Treatment of Perfluoroalkyl Contaminants in Groundwater. SERDP/ESTCP project ER-2424
- Development of a Novel Approach for In Situ Remediation of Pfc Contaminated Groundwater Systems. SERDP/ESTCP project ER-2425