Difference between revisions of "User:Admin/sandbox"
(Tag: Visual edit) |
|||
| Line 34: | Line 34: | ||
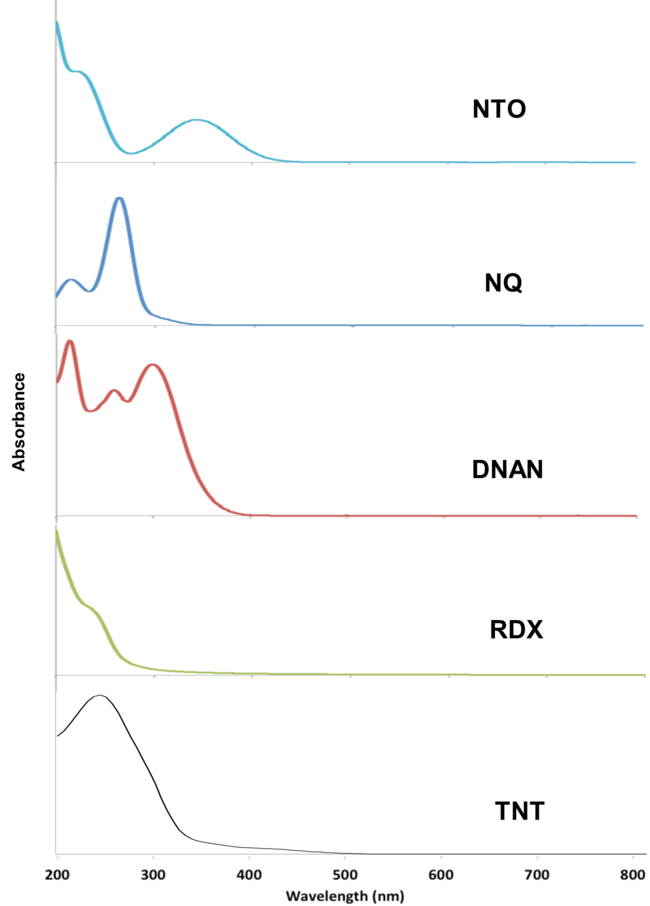
[[File: PhotolysisFig3.png | thumb | 650px | left | Figure 3. UV-Vis absorbance spectra for munitions compounds. Spectra over the wavelength range of 200-800 nm were obtained using a Jasco V-630 UV-Vis Spectrophotometer and quartz cuvettes, UV transparent to 200 nm (YeHui Instruments). Adapted from Taylor et al. (2017)<ref>Taylor, S., Becher, J., Beal, S., Ringelberg, D., Spanggord, R., and Dontsova, K., 2017. Photo-transformation of explosives and their constituents. In: The Environmental Aspects of Munitions Workshop, Joint Army-Navy-NASA-Air Force (JANNAF), Kansas City, MO, May 22, 2017.</ref>.]] | [[File: PhotolysisFig3.png | thumb | 650px | left | Figure 3. UV-Vis absorbance spectra for munitions compounds. Spectra over the wavelength range of 200-800 nm were obtained using a Jasco V-630 UV-Vis Spectrophotometer and quartz cuvettes, UV transparent to 200 nm (YeHui Instruments). Adapted from Taylor et al. (2017)<ref>Taylor, S., Becher, J., Beal, S., Ringelberg, D., Spanggord, R., and Dontsova, K., 2017. Photo-transformation of explosives and their constituents. In: The Environmental Aspects of Munitions Workshop, Joint Army-Navy-NASA-Air Force (JANNAF), Kansas City, MO, May 22, 2017.</ref>.]] | ||
For a chemical bond to break via direct photolysis, a molecule must absorb a photon with higher energy than the energy of the bond. The energy of photons in the UV-Vis range is similar to the bond energies of several single [[wikipedia:Covalent_bond|covalent bonds]] found in organic molecules<ref name=":1" />. Therefore, many of the bonds in TNT, DNAN, NTO, and NQ are susceptible to photolysis from sunlight exposure (Figure 4). | For a chemical bond to break via direct photolysis, a molecule must absorb a photon with higher energy than the energy of the bond. The energy of photons in the UV-Vis range is similar to the bond energies of several single [[wikipedia:Covalent_bond|covalent bonds]] found in organic molecules<ref name=":1" />. Therefore, many of the bonds in TNT, DNAN, NTO, and NQ are susceptible to photolysis from sunlight exposure (Figure 4). | ||
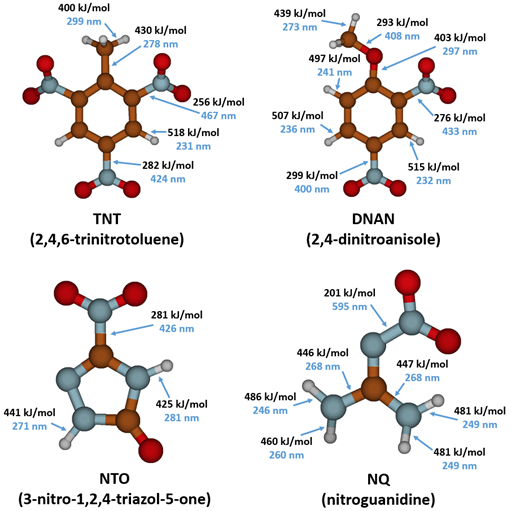
| − | [[File: PhotolysisFig4.png | thumb | 650px | left | Figure 4. Calculated bond energies (black) and corresponding photon wavelengths (blue, determined from Planck’s equation<ref name=":1" />) required to break the bonds in TNT, DNAN, NTO, and NQ (calculations by Dr. Diego Troya<ref> | + | [[File: PhotolysisFig4.png | thumb | 650px | left | Figure 4. Calculated bond energies (black) and corresponding photon wavelengths (blue, determined from Planck’s equation<ref name=":1" />) required to break the bonds in TNT, DNAN, NTO, and NQ (calculations by Dr. Diego Troya<ref>Dontsova, K., Taylor, S., Brusseau, M. L., Simunek, J., and Hunt, E., 2017. Influence of climate on dissolution and phototransformation of NTO and DNAN from insensitive munitions and their fate in soils. In: Poster, the SERDP-ESTCP symposium, Washington, D.C., November 28-30, 2017. The Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP).</ref>). Energies for bonds in the aromatic and heterocyclic rings are not shown because they exceed the maximum energy of the photons in sunlight reaching the Earth’s surface (500 kJ/mol or 239 nm).]] |
| − | Dontsova, K., Taylor, S., Brusseau, M. L., Simunek, J., and Hunt, E., 2017. Influence of climate on dissolution and phototransformation of NTO and DNAN from insensitive munitions and their fate in soils. In: Poster, the SERDP-ESTCP symposium, Washington, D.C., November 28-30, 2017. The Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP). | ||
| − | |||
Revision as of 20:24, 14 December 2021
I have installed SandboxLink extension that provides each user their own sandbox accessible through their personal menu bar (top right)
Munitions Constituents – Photolysis
Munitions compounds (MCs), including 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine (NQ), absorb light in the UV range and are therefore susceptible to photolysis on soil surfaces and in surface water. Photochemical reactions are important to consider when assessing the environmental impact of MCs since they can yield products that differ from their parent compounds in both toxicity and transport behavior. Quantum yield calculations can aid in predicting the photolysis rates and half-lives of MCs. The photolysis of MCs may be enhanced or inhibited in the presence of compounds that are also excited by UV irradiation. Munitions compounds (MCs), including 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine (NQ), absorb light in the UV range and are therefore susceptible to photolysis on soil surfaces and in surface water. Photochemical reactions are important to consider when assessing the environmental impact of MCs since they can yield products that differ from their parent compounds in both toxicity and transport behavior. Quantum yield calculations can aid in predicting the photolysis rates and half-lives of MCs. The photolysis of MCs may be enhanced or inhibited in the presence of compounds that are also excited by UV irradiation.
Related Article(s):
Contributor(s): Dr. Warren Kadoya
Key Resource(s):
- Environmental Organic Chemistry, Chapter 15: Direct Photolysis[1]
- Photochemical Degradation of Composition B and Its Components[2]
- Verification of RDX Photolysis Mechanism[3]
- Photochemical transformation of the insensitive munitions compound 2,4-dinitroanisole[4]
- Photo-transformation of aqueous nitroguanidine and 3-nitro-1,2,4-triazol-5-one: Emerging munitions compounds[5]
Introduction
Insensitive munitions, including IMX-101 and IMX-104, are replacing traditional explosives because they are less prone to accidental detonation and therefore safer for military personnel to handle. IMX-101, composed of 2,4-dinitroanisole (DNAN), 3-nitro-1,2,4-triazol-5-one (NTO), and nitroguanidine (NQ), will replace 2,4,6-trinitrotoluene (TNT) in artillery; IMX-104, composed of DNAN, NTO, and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), will replace Composition B (Comp B) in mortars[6]. As both traditional munitions compounds and these insensitive munitions compounds (collectively referred to as MCs) may be deposited onto firing ranges via incomplete detonation, understanding their environmental fate is of concern[7]. Phototransformation due to sunlight exposure is an important fate-controlling parameter for MCs and can occur on the surfaces of solid explosive particles, as shown in Figure 1, as well as in the aqueous phase following MC dissolution by rainwater. Furthermore, MC photolysis can be affected by the presence of natural organic matter and other compounds that are excited by sunlight.

Direct photolysis
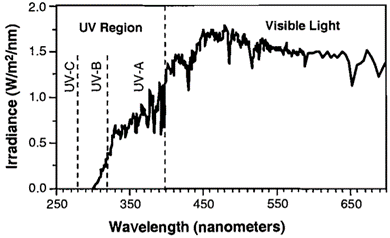
Compounds that absorb ultraviolet (UV, 100-400 nm) and/or visible (Vis, 400-800 nm) light can undergo direct photolysis from sunlight. Light is absorbed in discrete units, or photons, and the energy of these photons is indirectly proportional to the wavelength of the light. When a chemical molecule absorbs a photon, its ground state electrons may become excited. As the excited electrons return to the ground state, they may undergo a chemical reaction that results in transformation. Figure 2 shows that the UV range is further divided into UV-A (315-400 nm), UV-B (280-315 nm), and UV-C (100-280 nm). Because most of UV-C is filtered out by the Earth’s atmosphere, direct photolysis of compounds at the surface primarily involves UV-A and UV-B[9].
The MCs TNT, RDX, DNAN, NTO, and NQ may undergo direct photolysis since they all absorb light in the UV-Vis range (Figure 3). These graphs convey the probability that the compounds will absorb light at a given wavelength. The absorption maxima correspond to one or more electrons transitioning to an excited state.
For a chemical bond to break via direct photolysis, a molecule must absorb a photon with higher energy than the energy of the bond. The energy of photons in the UV-Vis range is similar to the bond energies of several single covalent bonds found in organic molecules[1]. Therefore, many of the bonds in TNT, DNAN, NTO, and NQ are susceptible to photolysis from sunlight exposure (Figure 4).
- ^ 1.0 1.1 1.2 Schwarzenbach, R.P., Gschwend, P.M., and Imboden, D.M., 2002. Chapter 15, Direct Photolysis. In: Schwarzenbach, R.P., Gschwend, P.M., and Imboden, D.M. (eds). Environmental Organic Chemistry. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc, pp. 611-654. doi:10.1002/0471649643.ch15
- ^ Pennington, J.C., Thorn, K.A., Co, L.G., MacMillan, D.K., Yost, S., and Laubscher, R.D., 2007. Photochemical Degradation of Composition B and Its Components. U.S. Army Engineer Research and Development Center (ERDC)/ Environmental Laboratory (EL) TR-07-16. Report
- ^ Peyton, G.R., LeFaivre, M.H., and Maloney, S.W., 1999. Verification of RDX photolysis mechanism. U.S. Army Engineer Research and Development Center (ERDC)/ Construction Engineering Research Laboratory (CERL) TR 99/93. Report
- ^ Rao, B., Wang, W., Cai, Q., Anderson, T., and Gu, B., 2013. Photochemical Transformation of The Insensitive Munitions Compound 2,4-Dinitroanisole. Science of The Total Environment, 443, pp. 692-699. doi: 10.1016/j.scitotenv.2012.11.033
- ^ Becher, J.B., Beal, S.A., Taylor, S., Dontsova, K., Wilcox, D.E., 2019. Photo-transformation of aqueous nitroguanidine and 3-nitro-1,2,4-triazol-5-one: Emerging munitions compounds. Chemosphere, 228, pp. 418-426. doi:10.1016/j.chemosphere.2019.04.131
- ^ BAE Systems, 2021. Making explosives safer
- ^ Pennington, J.C., Silverblatt, B., Poe, K., Hayes, C.A., and Yost, S, 2008. Explosive residues from low-order detonations of heavy artillery and mortar rounds. Soil and Sediment Contamination: An International Journal, 17(5), pp. 533-546. doi: 10.1080/15320380802306669
- ^ Dontsova, K., Taylor S., Pesce-Rodriguez, R., Brusseau, M., Arthur, J., Mark, N., Walsh, M., Lever, J., and Simunek, J., 2014. Dissolution of NTO, DNAN, and insensitive munitions formulations and their fates in soils. U.S. Army Engineer Research and Development Center (ERDC)/ Cold Region Research and Engineering Laboratory (CRREL) TR-14-23. Report
- ^ 9.0 9.1 Brennan, P., and Fedor, C., 1994. Sunlight, UV, & accelerated weathering. Q-Lab Corporation, Technical Bulletin LU-0822. Paper
- ^ Taylor, S., Becher, J., Beal, S., Ringelberg, D., Spanggord, R., and Dontsova, K., 2017. Photo-transformation of explosives and their constituents. In: The Environmental Aspects of Munitions Workshop, Joint Army-Navy-NASA-Air Force (JANNAF), Kansas City, MO, May 22, 2017.
- ^ Dontsova, K., Taylor, S., Brusseau, M. L., Simunek, J., and Hunt, E., 2017. Influence of climate on dissolution and phototransformation of NTO and DNAN from insensitive munitions and their fate in soils. In: Poster, the SERDP-ESTCP symposium, Washington, D.C., November 28-30, 2017. The Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP).