|
|
| (247 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| − | ==Transition of Aqueous Film Forming Foam (AFFF) Fire Suppression Infrastructure Impacted by Per and Polyfluoroalkyl Substances (PFAS)== | + | ==Munitions Constituents – Sample Extraction and Analytical Techniques== |
| − | [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)|Per and polyfluoroalkyl substances (PFAS)]] contained in [[wikipedia:Firefighting foam |Class B aqueous film-forming foams (AFFFs)]] are known to accumulate on wetted surfaces of many fire suppression systems after decades of exposure<ref name="LangEtAl2022">Lang, J.R., McDonough, J., Guillette, T.C., Storch, P., Anderson, J., Liles, D., Prigge, R., Miles, J.A.L., Divine, C., 2022. Characterization of per- and polyfluoroalkyl substances on fire suppression system piping and optimization of removal methods. Chemosphere, 308(Part 2), 136254. [https://doi.org/10.1016/j.chemosphere.2022.136254 doi: 10.1016/j.chemosphere.2022.136254] [[Media:LangEtAl2022.pdf | Open Access Article]]</ref>. When replacement PFAS-free firefighting formulations are added to existing infrastructure, PFAS can rebound from the wetted surfaces into the new formulations at high concentrations<ref name="RossStorch2020">Ross, I., and Storch, P., 2020. Foam Transition: Is It as Simple as "Foam Out / Foam In?". The Catalyst (Journal of JOIFF, The International Organization for Industrial Emergency Services Management), Q2 Supplement, 20 pages. [[Media:Catalyst_2020_Q2_Sup.pdf | Industry Newsletter]]</ref><ref>Kappetijn, K., 2023. Replacement of fluorinated extinguishing foam: When is clean clean enough? The Catalyst (Journal of JOIFF, The International Organization for Industrial Emergency Services Management), Q1 2023, pp. 31-33. [[Media:Catalyst_2023_Q1.pdf | Industry Newsletter]]</ref>. Effective methods are needed to properly transition to PFAS-free firefighting formulations in existing fire suppression infrastructure. Considerations in the transition process may include but are not limited to locating, identifying, and evaluating existing systems and AFFF, fire engineering evaluations, system prioritization, cost/downtime analyses, sampling and analysis, evaluation of risks and hazards to human health and the environment, transportation, and disposal. | + | Munitions Constituents, including [[Wikipedia: Insensitive munition | insensitive munitions]] IM), are a broad category of compounds and, in areas where manufactured or used, can be found in a variety of environmental matrices (waters, soil, and tissues). This presents an analytical challenge when a variety of these munitions are to be quantified. This article discusses sample extraction methods for each typical sample matrix (high level water, low level water, soil and tissue) as well as the accompanying [[Wikipedia: High-performance liquid chromatography | HPLC]]-UV analytical method for 27 compounds of interest (legacy munitions, insensitive munitions, and surrogates). |
| | + | |
| | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| | | | |
| | '''Related Article(s):''' | | '''Related Article(s):''' |
| − | *[[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]]
| + | |
| − | *[[PFAS Sources]]
| + | *[[Munitions Constituents]] |
| − | *[[PFAS Ex Situ Water Treatment]]
| |
| − | *[[Supercritical Water Oxidation (SCWO)]]
| |
| − | *[[PFAS Treatment by Electrical Discharge Plasma]] | |
| | | | |
| | '''Contributor(s):''' | | '''Contributor(s):''' |
| − | *Dr. Johnsie Ray Lang
| + | |
| − | *Dr. Jonathan Miles
| + | *Dr. Austin Scircle |
| − | *John Anderson
| |
| − | *Dr. Theresa Guillette
| |
| − | *[[Craig E. Divine, Ph.D., PG|Dr. Craig Divine]]
| |
| − | *[[Dr. Stephen Richardson]] | |
| | | | |
| | '''Key Resource(s):''' | | '''Key Resource(s):''' |
| − | *Department of Defense (DoD) performance standard for PFAS-free firefighting formulation: [https://media.defense.gov/2023/Jan/12/2003144157/-1/-1/1/MILITARY-SPECIFICATION-FOR-FIRE-EXTINGUISHING-AGENT-FLUORINE-FREE-FOAM-F3-LIQUID-CONCENTRATE-FOR-LAND-BASED-FRESH-WATER-APPLICATIONS.PDF Military Specification MIL-PRF-32725]<ref name="DoD2023">US Department of Defense, 2023. Performance Specification for Fire Extinguishing Agent, Fluorine-Free Foam (F3) Liquid Concentrate for Land-Based, Fresh Water Applications. Mil-Spec MIL-PRF-32725, 18 pages. [[Media: MilSpec32725.pdf | Military Specification Document]]</ref>
| |
| − | *[[Media:LangEtAl2022.pdf | Characterization of per- and polyfluoroalkyl substances on fire suppression system piping and optimization of removal methods]]<ref name="LangEtAl2022"/>
| |
| | | | |
| − | ==Introduction==
| + | *[https://www.epa.gov/sites/default/files/2015-07/documents/epa-8330b.pdf USEPA Method 8330B]<ref name= "8330B">United States Environmental Protection Agency (USEPA), 2006. EPA Method 8330B (SW-846) Nitroaromatics, Nitramines, and Nitrate Esters by High Performance Liquid Chromatography (HPLC), Revision 2. [https://www.epa.gov/esam/epa-method-8330b-sw-846-nitroaromatics-nitramines-and-nitrate-esters-high-performance-liquid USEPA Website] [[Media: epa-8330b.pdf | Method 8330B.pdf]]</ref> |
| − | [[File:LangFig1.png | thumb |400px|Figure 1. (A) Schematic of a typical PFAS molecule demonstrating the hydrophobic fluorinated tail in green and the hydrophilic charged functional group in blue, (B) a PFAS bilayer formed with the hydrophobic tails facing inward and the charged functional groups on the outside, and (C) multiple bilayers of PFAS assembled on the wetted surfaces of fire suppression piping.]]PFAS are a class of synthetic fluorinated compounds which are highly mobile and persistent within the environment<ref>Giesy, J.P., Kannan, K., 2001. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environmental Science and Technology 35(7), pp. 1339-1342. [https://doi.org/10.1021/es001834k doi: 10.1021/es001834k]</ref>. Due to the surfactant properties of PFAS, these compounds self-assemble at any solid-liquid interface forming resilient bilayers during prolonged exposure<ref>Krafft, M.P., Riess, J.G., 2015. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one. Chemosphere, 129, pp. 4-19. [https://doi.org/10.1016/j.chemosphere.2014.08.039 doi: 10.1016/j.chemosphere.2014.08.039]</ref>. Solid phase accumulation of PFAS has been proposed to be influenced by both [[wikipedia: Hydrophobic effect|hydrophobic]] and electrostatic interactions with fluorinated carbon chain length as the dominant feature influencing sorption<ref>Higgins, C.P., Luthy, R.G., 2006. Sorption of Perfluorinated Surfactants on Sediments. Environmental Science and Technology, 40(23), pp. 7251-7256. [https://doi.org/10.1021/es061000n doi: 10.1021/es061000n]</ref>. While the majority of previous research into solid phase sorption typically focused on water treatment applications or subsurface porous media<ref>Brusseau, M.L., 2018. Assessing the Potential Contributions of Additional Retention Processes to PFAS Retardation in the Subsurface. Science of the Total Environment, 613-614, pp. 176-185. [https://doi.org/10.1016/j.scitotenv.2017.09.065 doi: 10.1016/j.scitotenv.2017.09.065] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5693257/ Open Access Manuscript]</ref>, recently PFAS accumulations have been demonstrated on the wetted surfaces of fire suppression infrastructure exposed to aqueous film forming foam (AFFF)<ref name="LangEtAl2022"/> (see Figure 1).
| |
| − |
| |
| − | Fire suppression systems with potential PFAS impacts include fire fighting vehicles that carried AFFF and fixed suppression systems in buildings containing large amounts of flammable materials such as aircraft hangars (Figure 2). PFAS residue on the wetted surfaces of existing infrastructure can rebound into replacement PFAS-free firefighting formulations if not removed during the transition process<ref name="RossStorch2020"/>. Simple surface rinsing with water and low-pressure washing has been proven to be inefficient for removal of surface bound PFAS from piping and tanks that contained fluorinated AFFF<ref name="RossStorch2020"/>
| |
| − | [[File:LangFig2.png | thumb|left|600px|Figure 2. Fixed fire suppression system for an aircraft hangar, with storage tank on left and distribution piping on right.]]
| |
| | | | |
| − | In addition to proper methods for system cleaning to remove residual PFAS, transition to PFAS-free foam may also include consideration of compliance with state and federal regulations, selection of the replacement PFAS free firefighting formulation, a cost benefit analysis for replacement of the system components versus cleaning, and PFAS verification testing. Foam transition should be completed in a manner which minimizes the volume of waste generated as well as preventing any PFAS release into the environment.
| + | *Methods for simultaneous quantification of legacy and insensitive munition (IM) constituents in aqueous, soil/sediment, and tissue matrices<ref name="CrouchEtAl2020">Crouch, R.A., Smith, J.C., Stromer, B.S., Hubley, C.T., Beal, S., Lotufo, G.R., Butler, A.D., Wynter, M.T., Russell, A.L., Coleman, J.G., Wayne, K.M., Clausen, J.L., Bednar, A.J., 2020. Methods for simultaneous determination of legacy and insensitive munition (IM) constituents in aqueous, soil/sediment, and tissue matrices. Talanta, 217, Article 121008. [https://doi.org/10.1016/j.talanta.2020.121008 doi: 10.1016/j.talanta.2020.121008] [[Media: CrouchEtAl2020.pdf | Open Access Manuscript.pdf]]</ref> |
| | | | |
| − | ==PFAS Assembly on Solid Surfaces== | + | ==Introduction== |
| − | The self-assembly of [[Wikipedia: Amphiphile | amphiphilic]] molecules into supramolecular bilayers is a result of their structure and how it interacts with the bulk water of a solution. Single chain hydrocarbon based amphiphiles can form [[Wikipedia: Micelle | micelles]] under relatively dilute aqueous concentrations, however for hydrocarbon based surfactants the formation of more complex organized system such as [[Wikipedia: Vesicle (biology and chemistry) | vesicles]] is rarely seen, requiring double chain amphiphiles such as [[wikipedia: Phospholipid|phospholipids]]. Associations of single chain [[wikipedia: Ion#Anions_and_cations|cationic and anionic]] hydrocarbon based amphiphiles into stable supramolecular structures such as vesicles has however been demonstrated<ref>Fukuda, H., Kawata, K., Okuda, H., 1990. Bilayer-Forming Ion-Pair Amphiphiles from Single-Chain Surfactants. Journal of the American Chemical Society, 112(4), pp. 1635-1637. [https://doi.org/10.1021/ja00160a057 doi: 10.1021/ja00160a057]</ref>, with the ion pairing of the polar head groups mimicking the a double tail situation. The behavior of single chain [[wikipedia: Per-_and_polyfluoroalkyl_substances#Fluorosurfactants|fluorosurfactant]] amphiphiles has been demonstrated to be significantly different from similar hydrocarbon based analogues. Not only are [[Wikipedia: Critical micelle concentration | critical micelle concentrations (CMC)]] of fluorosurfactants typically two orders of magnitude lower than corresponding hydrocarbon surfactants but self-assembly can occur even when fluorosurfactants are dispersed at low concentrations significantly below the CMC in water and other solvents<ref name="Krafft2006">Krafft, M.P., 2006. Highly fluorinated compounds induce phase separation in, and nanostructuration of liquid media. Possible impact on, and use in chemical reactivity control. Journal of Polymer Science Part A: Polymer Chemistry, 44(14), pp. 4251-4258. [https://doi.org/10.1002/pola.21508 doi: 10.1002/pola.21508] [[Media:Krafft2006.pdf | Open Access Article]]</ref>. The assembly of fluorinated amphiphiles structurally similar to those found in AFFF have been shown to readily form stable, complex structures including vesicles, fibers, and globules at concentrations as low as 0.5% w/v in contrast to their hydrocarbon analogues which remained fluid at 30% w/v<ref>Krafft, M.P., Guilieri, F., Riess, J.G., 1993. Can Single-Chain Perfluoroalkylated Amphiphiles Alone form Vesicles and Other Organized Supramolecular Systems? Angewandte Chemie International Edition in English, 32(5), pp. 741-743. [https://doi.org/10.1002/anie.199307411 doi: 10.1002/anie.199307411]</ref><ref name="KrafftEtAl_1994">Krafft, M.P., Guilieri, F., Riess, J.G., 1994. Supramolecular assemblies from single chain perfluoroalkylated phosphorylated amphiphiles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 84(1), pp. 113-119. [https://doi.org/10.1016/0927-7757(93)02681-4 doi: 10.1016/0927-7757(93)02681-4]</ref>.
| + | [[File: ScircleFig1.png | thumb | 400px | Figure 1. Primary Method labeled chromatograms]] |
| − | | + | [[File: ScircleFig2.png | thumb | 400px | Figure 2. Secondary Method labeled chromatograms]] |
| − | Krafft found that fluorinated amphiphiles formed bilayer membranes with phospholipids, and that the resulting vesicles were more stable than those made of phospholipids alone<ref name="KrafftEtAl_1998">Krafft, M.P., Riess, J.G., 1998. Highly Fluorinated Amphiphiles and Collodial Systems, and their Applications in the Biomedical Field. A Contribution. Biochimie, 80(5-6), pp. 489-514. [https://doi.org/10.1016/S0300-9084(00)80016-4 doi: 10.1016/S0300-9084(00)80016-4]</ref>. The similarities in amphiphilic properties between phospholipids and the hydrocarbon-based surfactants in AFFF suggests that bilayer vesicles may form between these and the fluorosurfactants also present in the concentrate. Krafft demonstrated that both the permeability of resulting mixed vesicles and their propensity to fuse with each other at increasing ionic strength was reduced as a result of the creation of an inert hydrophobic and [[wikipedia: Lipophobicity|lipophobic]] film within the membrane, and also suggested that the fluorinated amphiphiles increased [[Wikipedia: van der Waals force | van der Waals interactions]] in the hydrocarbon region<ref name="KrafftEtAl_1998"/>. Thus this low permeability may allow vesicles formed by the surfactants present in AFFF to act as long term repositories of PFAS not only as part of the bilayer itself but also solvated within the vesicle. This prediction is supported by the observation that supramolecular structures formed from single chain fluorinated amphiphiles have been demonstrated to be stable at elevated temperature (15 min at 121°C) and have been shown to be stable over periods of months, even increasing in size over time when stored at environmentally relevant temperatures<ref name="KrafftEtAl_1994"/>.
| + | The primary intention of the analytical methods presented here is to support the monitoring of legacy and insensitive munitions contamination on test and training ranges, however legacy and insensitive munitions often accompany each other at demilitarization facilities, manufacturing facilities, and other environmental sites. Energetic materials typically appear on ranges as small, solid particulates and due to their varying functional groups and polarities, can partition in various environmental compartments<ref>Walsh, M.R., Temple, T., Bigl, M.F., Tshabalala, S.F., Mai, N. and Ladyman, M., 2017. Investigation of Energetic Particle Distribution from High‐Order Detonations of Munitions. Propellants, Explosives, Pyrotechnics, 42(8), pp. 932-941. [https://doi.org/10.1002/prep.201700089 doi: 10.1002/prep.201700089]</ref>. To ensure that contaminants are monitored and controlled at these sites and to sustainably manage them a variety of sample matrices (surface or groundwater, process waters, soil, and tissues) must be considered. (Process water refers to water used during industrial manufacturing or processing of legacy and insensitive munitions.) Furthermore, additional analytes must be added to existing methodologies as the usage of IM compounds changes and as new degradation compounds are identified. Of note, relatively new IM formulations containing NTO, DNAN, and NQ are seeing use in [[Wikipedia: IMX-101 | IMX-101]], IMX-104, Pax-21 and Pax-41 (Table 1)<ref>Mainiero, C. 2015. Picatinny Employees Recognized for Insensitive Munitions. U.S. Army, Picatinny Arsenal Public Affairs. [https://www.army.mil/article/148873/picatinny_employees_recognized_for_insensitive_munitions Open Access Press Release]</ref><ref>Frem, D., 2022. A Review on IMX-101 and IMX-104 Melt-Cast Explosives: Insensitive Formulations for the Next-Generation Munition Systems. Propellants, Explosives, Pyrotechnics, 48(1), e202100312. [https://doi.org/10.1002/prep.202100312 doi: 10.1002/prep.202100312]</ref>. |
| − | | |
| − | Formation of complex structures at relatively low solute concentrations requires the monomer molecules to be well ordered to maintain tight packing in the supramolecular structure<ref>Ringsdorf, H., Schlarb, B., Venzmer, J., 1988. Molecular Architecture and Function of Polymeric Oriented Systems: Models for the Study of Organization, Surface Recognition, and Dynamics of Biomembranes. Angewandte Chemie International Edition in English, 27(1), pp. 113-158. [https://doi.org/10.1002/anie.198801131 doi: 10.1002/anie.198801131]</ref>. This order results from electrostatic forces, [[wikipedia: Hydrogen bond|hydrogen bonding]], and in the case of fluorinated amphiphiles, hydrophobic interactions. The geometry of the amphiphile also potentially contributes to the type of supramolecular aggregation<ref>Israelachvili, J.N., Mitchell, D.J., Ninham, B.W., 1976. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics, 72, pp. 1525-1568. [https://doi.org/10.1039/F29767201525 doi: 10.1039/F29767201525]</ref>. Surfactants which adopt a conical shape (such as a typical hydrocarbon based surfactant with a large polar head group and a single alkyl chain as a tail) tend to form micelles more easily. Increasing the bulk of the tail makes the surfactant more cylindrically shaped which makes assembly into bilayers more likely.
| |
| − | | |
| − | Perfluoroalkyl chains are significantly more bulky than their hydrocarbon based analogues both in cross sectional area (28-30 Å<sup>2</sup> versus 20 Å<sup>2</sup>, respectively) and mean volume (CF<sub>2</sub> and CF<sub>3</sub> estimated as 38 Å<sup>3</sup> and 92 Å<sup>3</sup> compared to 27 Å<sup>3</sup> and 54 Å<sup>3</sup> for CH<sub>2</sub> and CH<sub>3</sub>)<ref name="KrafftEtAl_1998"/><ref name="Krafft2006"/>. Structural studies on linear PFOS have shown that the molecule adopts an unusual helical structure<ref>Erkoç, Ş., Erkoç, F., 2001. Structural and electronic properties of PFOS and LiPFOS. Journal of Molecular Structure: THEOCHEM, 549(3), pp. 289-293. [https://doi.org/10.1016/S0166-1280(01)00553-X doi:10.1016/S0166-1280(01)00553-X]</ref><ref name="TorresEtAl2009">Torres, F.J., Ochoa-Herrera, V., Blowers, P., Sierra-Alvarez, R., 2009. Ab initio study of the structural, electronic, and thermodynamic properties of linear perfluorooctane sulfonate (PFOS) and its branched isomers. Chemosphere 76(8), pp. 1143-1149. [https://doi.org/10.1016/j.chemosphere.2009.04.009 doi: 10.1016/j.chemosphere.2009.04.009]</ref> in aqueous and solvent phases to alleviate [[wikipedia: Steric_effects#Steric_hindrance|steric hindrance]]. This arrangement results from the carbon chain starting in the planar all anti [[wikipedia:Conformational isomerism|conformation]] and then successively twisting all the CC-CC dihedrals by 15°-20° in the same direction<ref>Abbandonato, G., Catalano, D., Marini, A., 2010. Aggregation of Perfluoroctanoate Salts Studied by <sup>19</sup>F NMR and DFT Calculations: Counterion Complexation, Poly(ethylene glycol) Addition, and Conformational Effects. Langmuir 26(22), pp. 16762-16770. [https://doi.org/10.1021/la102578k doi: 10.1021/la102578k].</ref>. The conformation also minimizes the electrostatic repulsion between fluorine atoms bonded to the same side of the carbon backbone by maximizing the interatomic distances between them<ref name="TorresEtAl2009"/>.
| |
| − | | |
| − | A consequence of the helical structure is that there is limited carbon-carbon bond rotation within the perfluoroalkyl chain giving them increased rigidity compared to alkyl chains<ref>Barton, S.W., Goudot, A., Bouloussa, O., Rondelez, F., Lin, B., Novak, F., Acero, A., Rice, S., 1992. Structural transitions in a monolayer of fluorinated amphiphile molecules. The Journal of Chemical Physics, 96(2), pp. 1343-1351. [https://doi.org/10.1063/1.462170 doi: 10.1063/1.462170]</ref>. The bulkiness of the perfluoroalkyl chain confers a cylindrical shape on the fluorosurfactant amphiphile and therefore favors the formation of bilayers and vesicles the aggregation of which is further assisted by the rigidity of the molecules which allow close packing in the supramolecular structure. Fluorosurfactants therefore cannot be regarded as more hydrophobic analogues of hydrogenated surfactants. Their self-assembly behavior is characterized by a strong tendency to form vesicles and lamellar phases rather than micelles, due to the bulkiness and rigidity of the perfluoroalkyl chain that tends to decrease the curvature of the aggregates they form in solution<ref>Barton, C.A., Butler, L.E., Zarzecki, C.J., Flaherty, J., Kaiser, M., 2006. Characterizing Perfluorooctanoate in Ambient Air near the Fence Line of a Manufacturing Facility: Comparing Modeled and Monitored Values. Journal of the Air and Waste Management Association, 56, pp. 48-55. [https://doi.org/10.1080/10473289.2006.10464429 doi: 10.1080/10473289.2006.10464429] [https://www.tandfonline.com/doi/epdf/10.1080/10473289.2006.10464429?needAccess=true Open Access Article]</ref>. The larger tail cross section of fluorinated compared to hydrogenated amphiphiles tends to favor the formation of aggregates with lesser surface curvature, therefore rather than micelles they form bilayer membranes, vesicles, tubules and fibers<ref>Krafft, M.P., Guilieri, F., Riess, J.G., 1993. Can Single-Chain Perfluoroalkylated Amphiphiles Alone form Vesicles and Other Organized Supramolecular Systems? Angewandte Chemie International Edition in English, 32(5), pp. 741-743. [https://doi.org/10.1002/anie.199307411 doi: 10.1002/anie.199307411]</ref><ref>Furuya, H., Moroi, Y., Kaibara, K., 1996. Solid and Solution Properties of Alkylammonium Perfluorocarboxylates. The Journal of Physical Chemistry, 100(43), pp. 17249-17254. [https://doi.org/10.1021/jp9612801 doi: 10.1021/jp9612801]</ref><ref>Giulieri, F., Krafft, M.P., 1996. Self-organization of single-chain fluorinated amphiphiles with fluorinated alcohols. Thin Solid Films, 284-285, pp. 195-199. [https://doi.org/10.1016/S0040-6090(95)08304-9 doi: 10.1016/S0040-6090(95)08304-9]</ref><ref>Gladysz, J.A., Curran, D.P., Horvath, I.T., 2004. Handbook of Fluorous Chemistry. WILEY-VCH Verlag GmbH & Co. KGaA,, Weinheim, Germany. ISBN: 3-527-30617-X</ref>. Rojas ''et al.'' (2002) demonstrated that perfluorooctyl sulphonamide formed a contiguous bilayer at 50 mg/L with self-assembled aggregates present at concentrations as low as 10 mg/L<ref name="RojasEtAl2002">Rojas, O.J., Macakova, L., Blomberg, E., Emmer, A., and Claesson, P.M., 2002. Fluorosurfactant Self-Assembly at Solid/Liquid Interfaces. Langmuir, 18(21), pp. 8085-8095. [https://doi.org/10.1021/la025989c doi: 10.1021/la025989c]</ref>.
| |
| | | | |
| − | ==Thermodynamics of PFAS Accumulations on Solid Surfaces== | + | Sampling procedures for legacy and insensitive munitions are identical and utilize multi-increment sampling procedures found in USEPA Method 8330B Appendix A<ref name= "8330B"/>. Sample hold times, subsampling and quality control requirements are also unchanged. The key differences lie in the extraction methods and instrumental methods. Briefly, legacy munitions analysis of low concentration waters uses a single cartridge reverse phase [[Wikipedia: Solid-phase extraction | SPE]] procedure, and [[Wikipedia: Acetonitrile | acetonitrile]] (ACN) is used for both extraction and [[Wikipedia: Elution | elution]] for aqueous and solid samples<ref name= "8330B"/><ref>United States Environmental Protection Agency (USEPA), 2007. EPA Method 3535A (SW-846) Solid-Phase Extraction (SPE), Revision 1. [https://www.epa.gov/esam/epa-method-3535a-sw-846-solid-phase-extraction-spe USEPA Website] [[Media: epa-3535a.pdf | Method 3535A.pdf]]</ref>. An [[Wikipedia: High-performance_liquid_chromatography#Isocratic_and_gradient_elution | isocratic]] separation via reversed-phase C-18 column with 50:50 methanol:water mobile phase or a C-8 column with 15:85 isopropanol:water mobile phase is used to separate legacy munitions<ref name= "8330B"/>. While these procedures are sufficient for analysis of legacy munitions, alternative solvents, additional SPE cartridges, and a gradient elution are all required for the combined analysis of legacy and insensitive munitions. |
| − | The thermodynamics of formation of amphiphiles into supramolecular species requires consideration of both hydrophobic and hydrophilic interactions resulting from the amphoteric nature of the molecule. The hydrophilic portions of the molecule are driven to maximize their solvation interaction with as many water molecules as possible, whereas the hydrophobic portions of the molecule are driven to aggregate together thus minimizing interaction with the bulk water. Both of these processes change the enthalpy and entropy of the system.
| |
| | | | |
| − | In aqueous solution, the hydrophilic portions of an amphiphile form hydrogen bonds (4 - 120 kJ/mol) and van der Waals interactions (<5 kJ/mol) with water molecules and surfaces, and electrostatic interactions (5 – 300 kJ/mol) can also occur where the amphiphile is ionic<ref name="LombardoEtAl2015">Lombardo, D., Kiselev, M.A., Magazù, S., Calandra, P., 2015. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Advances in Condensed Matter Physics, vol. 2015, article ID 151683, 22 pages. [https://doi.org/10.1155/2015/151683 doi: 10.1155/2015/151683] [[Media: LombardoEtAl2015.pdf | Open Access Article]]</ref>. These interactions, although weak compared to intramolecular covalent bonds within a molecule are energetically favorable and increase the enthalpy of the combined solute-solvent system. Thus, the hydrophilic portion of an amphiphile will look to maximize enthalpic gain through hydrogen bond interactions with the bulk water.
| + | Previously, analysis of legacy and insensitive munitions required multiple analytical techniques, however the methods presented here combine the two munitions categories resulting in an HPLC-UV method and accompanying extraction methods for a variety of common sample matrices. A secondary HPLC-UV method and a HPLC-MS method were also developed as confirmatory methods. The methods discussed in this article were validated extensively by single-blind round robin testing and subsequent statistical treatment as part of ESTCP [https://serdp-estcp.mil/projects/details/d05c1982-bbfa-42f8-811d-51b540d7ebda ER19-5078]. Wherever possible, the quality control criteria in the Department of Defense Quality Systems Manual for Environmental Laboratories were adhered to<ref>US Department of Defense and US Department of Energy, 2021. Consolidated Quality Systems Manual (QSM) for Environmental Laboratories, Version 5.4. 387 pages. [https://www.denix.osd.mil/edqw/denix-files/sites/43/2021/10/QSM-Version-5.4-FINAL.pdf Free Download] [[Media: QSM-Version-5.4.pdf | QSM Version 5.4.pdf]]</ref>. Analytes included in these methods are found in Table 1. |
| | | | |
| − | The hydrophobic portion of an amphiphile cannot form hydrogen bonds with the bulk solution, and its presence disrupts the hydrogen bond interactions between individual water molecules within the bulk water matrix. This disruption lowers the entropy of the system by reducing the degrees of translational rotational freedom available to the bulk water. The second law of thermodynamics dictates that a system will arrange itself to maximize its entropy. With hydrophobic species this can be achieved by their spontaneous aggregation, as the reduction in solution entropy of the aggregated system is less than that which would occur if the component parts were solvated individually. These hydrophobic and hydrophilic interactions are weak, and the individual entropy gain per amphiphile upon aggregation is very small. However, taken together the overall effect on the entropy of the aggregate is sufficient to maintain it in solution, and moreover these interactions make the aggregates resistant to minor perturbations while retaining the reversibility of the self-assembled structure<ref name="LombardoEtAl2015"/>. | + | The chromatograms produced by the primary and secondary HPLC-UV methods are shown in Figure 1 and Figure 2, respectively. Chromatograms for each detector wavelength used are shown (315, 254, and 210 nm). |
| | | | |
| − | ==Regulatory Drivers for Transition to PFAS Free Firefighting Formulations== | + | ==Extraction Methods== |
| − | Regulations restricting the use and release of PFAS are being proposed and promulgated worldwide, with several enacted regulations addressing the use of aqueous film forming foams (AFFF) containing PFAS<ref name="Queensland2016">Queensland (Australia) Department of Environment and Heritage Protection, 2016. Operational Policy - Environmental Management of Firefighting Foam. 16 pages. [https://environment.des.qld.gov.au/assets/documents/regulation/firefighting-foam-policy.pdf Free Download]</ref><ref>U.S. Congress, 2019. S.1790 - National Defense Authorization Act for Fiscal Year 2020. United States Library of Congress. [https://www.congress.gov/bill/116th-congress/senate-bill/1790 Text and History of Law].</ref><ref>Arizona State Legislature, 2019. Title 36, Section 1696. Firefighting foam; prohibited uses; exception; definitions. [https://www.azleg.gov/viewdocument/?docName=https://www.azleg.gov/ars/36/01696.htm Text of Law]</ref><ref>California Legislature, 2020. Senate Bill No. 1044, Chapter 308, Firefighting equipment and foam: PFAS chemicals. [https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB1044 Text and History of Law]</ref><ref>Arkansas General Assembly, 2021. An Act Concerning the Use of Certain Chemicals in Firefighting Foam; and for Other Purposes. Act 315, State of Arkansas. [https://trackbill.com/bill/arkansas-house-bill-1351-concerning-the-use-of-certain-chemicals-in-firefighting-foam/2008913/ Text and History of Law].</ref><ref>Espinosa, Summers, Kelly, J., Statler, Hansen, Young, 2021. Amendment to Fire Prevention and Control Act. House Bill 2722. West Virginia Legislature. [https://trackbill.com/bill/west-virginia-house-bill-2722-prohibiting-the-use-of-class-b-fire-fighting-foam-for-testing-purposes-if-the-foam-contains-a-certain-class-of-fluorinated-organic-chemicals/2047674/ Text and History of Law]</ref><ref>Louisiana Legislature, 2021. Act No. 232. [https://trackbill.com/bill/louisiana-house-bill-389-fire-protect-fire-marshal-provides-relative-to-the-discharge-or-use-of-class-b-fire-fighting-foam-containing-fluorinated-organic-chemicals/2092535/ Text and History of Law]</ref><ref>Vermont Legislature, 2021b. Act No. 36, PFAS in Class B Firefighting Foam. [https://trackbill.com/bill/vermont-senate-bill-20-an-act-relating-to-restrictions-on-perfluoroalkyl-and-polyfluoroalkyl-substances-and-other-chemicals-of-concern-in-consumer-products/1978963/ History and Text of Law]</ref>. In addition to regulated usage, firefighting formulation users are transitioning to PFAS-free firefighting formulations to reduce environmental liability in the event of a release, to reduce the cost of expensive containment systems and management of generated waste streams, and to avoid reputational damage. In 2016, Queensland, Australia was one of the first governments to ban PFAS use in firefighting foam<ref name="Queensland2016"/>. The US 2020 National Defense Authorization Act specifies immediate prohibition of controlled releases of AFFF containing PFAS and requires the Secretary of the Navy to publish a specification for PFAS-free firefighting formulation use and ensure it is available for use by the Department of Defense (DoD) by October 1, 2023<ref>U.S. Congress, 2021. S.2792 - National Defense Authorization Act for Fiscal Year 2021. United States Library of Congress. [https://www.congress.gov/bill/117th-congress/senate-bill/2792/ Text and History of Law].</ref>. The National Fire Protection Association (NFPA) recently removed the requirement for AFFF containing PFAS from their Standard on Aircraft Hangars and added two new chapters to allow users to determine if AFFF containing PFAS is needed at their facility<ref name="NFPA2022">National Fire Protection Association (NFPA), 2022. Codes and Standards, 409: Standard on Aircraft Hangars. [https://www.nfpa.org/codes-and-standards/4/0/9/409?l=42 NFPA Website]</ref>.
| + | ===High Concentration Waters (> 1 ppm)=== |
| | + | Aqueous samples suspected to contain the compounds of interest at concentrations detectable without any extraction or pre-concentration are suitable for analysis by direct injection. The method deviates from USEPA Method 8330B by adding a pH adjustment and use of MeOH rather than ACN for dilution<ref name= "8330B"/>. The pH adjustment is needed to ensure method accuracy for ionic compounds (like NTO or PA) in basic samples. A solution of 1% HCl/MeOH is added to both acidify and dilute the samples to a final acid concentration of 0.5% (vol/vol) and a final solvent ratio of 1:1 MeOH/H<sub>2</sub>O. The direct injection samples are then ready for analysis. |
| | | | |
| − | ==Selection of Replacement PFAS Free Firefighting Formulations== | + | ===Low Concentration Waters (< 1 ppm)=== |
| − | Since they first entered the market in the 2000s, the operational capabilities of PFAS free firefighting formulations have grown<ref>Allcorn, M., Bluteau, T., Corfield, J., Day, G., Cornelsen, M., Holmes, N.J.C., Klein, R.A., McDowall, J.G., Olsen, K.T., Ramsden, N., Ross, I., Schaefer, T.H., Weber, R., Whitehead, K., 2018. Fluorine-Free Firefighting Foams (3F) – Viable Alternatives to Fluorinated Aqueous Film-Forming Foams (AFFF). White Paper prepared for the IPEN by members of the IPEN F3 Panel and associates, POPRC-14, Rome. [https://ipen.org/sites/default/files/documents/IPEN_F3_Position_Paper_POPRC-14_12September2018d.pdf Free Download].</ref> and numerous companies are now manufacturing and delivering PFAS-free firefighting formulations for fixed systems and AFFF vehicles<ref>Ansul (Company), Ansul NFF-331 3%x3% Non-Fluorinated Foam Concentrate (Commercial Product). [https://docs.johnsoncontrols.com/specialhazards/api/khub/documents/1nbeVfynU1IW~eJcCOA0Bg/content Product Data Sheet].</ref><ref>BioEx (Company), Ecopol A+ (Commercial Product). [https://www.bio-ex.com/en/our-products/product/ecopol-aplus/ Website]</ref><ref>National Foam (Company), 2020. Avio F3 Green KHC 3%, Fluorine Free Foam Concentrate (Commercial Product). [https://nationalfoam.com/wp-content/uploads/sites/4/NMS515-Avio-Green-KHC-3-FF.pdf Safety Data Sheet]</ref>. Key factors in the selection of a PFAS-free firefighting formulations product are compatibility of the new formulation with the existing system (as confirmed by a fire protection engineer) and environmental certifications (i.e., verifying the absence of organic fluorine or PFAS or the absence of other non-fluorine environmental contaminants).
| + | Aqueous samples suspected to contain the compounds of interest at low concentrations require extraction and pre-concentration using solid phase extraction (SPE). The SPE setup described here uses a triple cartridge setup shown in '''Figure 3'''. Briefly, the extraction procedure loads analytes of interest onto the cartridges in this order: Strata<sup><small>TM</small></sup> X, Strata<sup><small>TM</small></sup> X-A, and Envi-Carb<sup><small>TM</small></sup>. Then the cartridge order is reversed, and analytes are eluted via a two-step elution, resulting in 2 extracts (which are combined prior to analysis). Five milliliters of MeOH is used for the first elution, while 5 mL of acidified MeOH (2% HCl) is used for the second elution. The particular SPE cartridges used are noncritical so long as cartridge chemistries are comparable to those above. |
| | | | |
| − | In January 2023, the US Department of Defense (DoD) published the Performance Specification for Fire Extinguishing Agent, Fluorine-Free Foam (F3) Liquid Concentrate for Land-Based, Fresh Water Applications<ref name="DoD2023"/>. This Military Performance Specification (Mil-Spec) allows PFAS free firefighting formulations to be certified as meeting certain standardized operational goals for use in military settings. In addition to Mil-Spec requirements, PFAS free firefighting formulations can also be certified through Underwriters Laboratories Standard for Safety, Foam Equipment and Liquid Concentrates, UL 162, which requires the new firefighting formulations be investigated for suitability and compatibility with the specific equipment with which they are intended to be used<ref>Underwriters Laboratories Inc., 2018. UL162, UL Standard for Safety, Foam Equipment and Liquid Concentrates, 8th Edition, Revised 2022. 40 pages. [https://global.ihs.com/doc_detail.cfm?document_name=UL%20162&item_s_key=00096960 Website]</ref>. Several PFAS-free foams have been certified under various parts of EN1568, the European Standard which specifies the necessary foam properties and performance requirements<ref>CSN EN 1568-1 ed. 2, 2018. Fire extinguishing media - Foam concentrates - Part 1: Specification for medium expansion foam concentrates for surface application to water-immiscible liquids. 48 pages. [https://www.en-standard.eu/csn-en-1568-1-ed-2-fire-extinguishing-media-foam-concentrates-part-1-specification-for-medium-expansion-foam-concentrates-for-surface-application-to-water-immiscible-liquids/ European Standards Website.]</ref>. Both ESTCP and SERDP have supported (and continue to support) the development and field validation of PFAS free firefighting formulations (e.g. WP22-7456, WP21-3461, WP20-1535, WP20-1524). Both the US Federal Aviation Administration (FAA) and National Fire Protection Association (NFPA) have performed a variety of foam certification tests on numerous PFAS-free firefighting formulations<ref>Back, G.G., Farley, J.P., 2020. Evaluation of the Fire Protection Effectiveness of Fluorine Free Firefighting Foams. National Fire Protection Association, Fire Protection Research Foundation. [https://www.iafc.org/docs/default-source/1safehealthshs/effectivenessofflourinefreefoam.pdf Free Download].</ref><ref>Casey, J., Trazzi, D., 2022. Fluorine-Free Foam Testing. Federal Aviation Administration (FAA) Final Report. [https://www.airporttech.tc.faa.gov/DesktopModules/EasyDNNNews/DocumentDownload.ashx?portalid=0&moduleid=3682&articleid=2882&documentid=3054 Open Access Article]</ref>.
| + | ===Soils=== |
| | + | Soil collection, storage, drying and grinding procedures are identical to the USEPA Method 8330B procedures<ref name= "8330B"/>; however, the solvent extraction procedure differs in the number of sonication steps, sample mass and solvent used. A flow chart of the soil extraction procedure is shown in '''Figure 4'''. Soil masses of approximately 2 g and a sample to solvent ratio of 1:5 (g/mL) are used for soil extraction. The extraction is carried out in a sonication bath chilled below 20 ⁰C and is a two-part extraction, first extracting in MeOH (6 hours) followed by a second sonication in 1:1 MeOH:H<sub>2</sub>O solution (14 hours). The extracts are centrifuged, and the supernatant is filtered through a 0.45 μm PTFE disk filter. |
| | | | |
| − | ==Selection of Flushing Agent==
| + | The solvent volume should generally be 10 mL but if different soil masses are required, solvent volume should be 5 mL/g. The extraction results in 2 separate extracts (MeOH and MeOH:H<sub>2</sub>O) that are combined prior to analysis. |
| − | General industry guidance has typically recommended several rinses with water to remove PFAS from impacted equipment. Owing to the unique physical and chemical properties of PFAS, the use of room temperature water to remove PFAS from impacted equipment has not been very effective. To address these recalcitrant accumulations, companies are developing new methods to remove self-assembled PFAS bilayers from existing fire-fighting infrastructure so that it can be successfully transitioned to PFAS-free formulations. Arcadis developed a non-toxic cleaning agent, Fluoro Fighter<sup>TM</sup>, which has been demonstrated to be effective for removal of PFAS from equipment by disrupting the accumulated layers of PFAS coating the AFFF-wetted surfaces.
| |
| | | | |
| − | Laboratory studies have supported the optimization of this PFAS removal method in fire suppression system piping obtained from a commercial airport hangar in Sydney, Australia<ref name="LangEtAl2022"/>. Prior to removal from the hangar, the stainless-steel pipe held PFAS-containing AFFF for more than three decades. Results indicated that Fluoro Fighter<sup>TM</sup>, as well as flushing at elevated temperatures, removed more surface associated PFAS in comparison to equivalent extractions using methanol or water at room temperature. ESTCP has supported (and continues to support) the development and field validation of best practices for methodologies to clean foam delivery systems (e.g. ER20-5364, ER20-5361, ER20-5369, ER21-7229).
| + | ===Tissues=== |
| | + | Tissue matrices are extracted by 18-hour sonication using a ratio of 1 gram of wet tissue per 5 mL of MeOH. This extraction is performed in a sonication bath chilled below 20 ⁰C and the supernatant (MeOH) is filtered through a 0.45 μm PTFE disk filter. |
| | | | |
| − | ==PFAS Verification Testing==
| + | Due to the complexity of tissue matrices, an additional tissue cleanup step, adapted from prior research, can be used to reduce interferences<ref name="RussellEtAl2014">Russell, A.L., Seiter, J.M., Coleman, J.G., Winstead, B., Bednar, A.J., 2014. Analysis of munitions constituents in IMX formulations by HPLC and HPLC-MS. Talanta, 128, pp. 524–530. [https://doi.org/10.1016/j.talanta.2014.02.013 doi: 10.1016/j.talanta.2014.02.013]</ref><ref name="CrouchEtAl2020"/>. The cleanup procedure uses small scale chromatography columns prepared by loading 5 ¾” borosilicate pipettes with 0.2 g activated silica gel (100–200 mesh). The columns are wetted with 1 mL MeOH, which is allowed to fully elute and then discarded prior to loading with 1 mL of extract and collecting in a new amber vial. After the extract is loaded, a 1 mL aliquot of MeOH followed by a 1 mL aliquot of 2% HCL/MeOH is added. This results in a 3 mL silica treated tissue extract. This extract is vortexed and diluted to a final solvent ratio of 1:1 MeOH/H<sub>2</sub>O before analysis. |
| − | In general, PFAS sampling techniques used to support firefighting formulation transition activities are consistent with conventional sampling techniques used in the environmental industry, but special consideration is made regarding high concentration PFAS materials, elevated detection levels, cross-contamination potential, precursor content, and matrix interferences. The analytical method selected should be appropriate for the regulatory requirements in the site area.
| |
| | | | |
| − | ==Rinsate Treatment== | + | ==HPLC-UV and MS Methods== |
| − | Numerous technologies for treatment of PFAS-impacted water sources, including rinsates, have been and are currently being developed. These include separation technologies such as foam fractionation, nanofiltration, sorbents/flocculants, ion exchange resins, reverse osmosis. and destructive technologies such as [PFAS Treatment by Electrical Discharge Plasma |enhanced contact plasma], [Supercritical Water Oxidation (SCWO |supercritical water oxidation (SCWO)], electrochemical oxidation, hydrothermal alkaline treatment, and sonolysis. Many of these technologies have rapidly developed from bench-scale (e.g., microcosms, columns, single reactors) to commercially available field-scale units capable of managing PFAS-impacted waters of varying waste volumes and PFAS compositions and concentrations. Ongoing field research continues to improve the treatment efficiency, reliability, and versatility of these technologies, both individually and as coupled treatment solutions (e.g., treatment train). ESTCP has supported (and continues to support) the development and field validation of separation and destructive technologies for treatment of PFAS-impacted water sources, including rinsates (e.g. ER20-5370, ER20-5369, ER20-5350, ER20-5355).
| + | The Primary HPLC method uses a Phenomenex Synergi 4 µm Hydro-RP column (80Å, 250 x 4.6 mm), or comparable, and is based on both the HPLC method found in USEPA 8330B and previous work<ref name= "8330B"/><ref name="RussellEtAl2014"/><ref name="CrouchEtAl2020"/>. This separation relies on a reverse phase column and uses a gradient elution, shown in Table 2. Depending on the analyst’s needs and equipment availability, the method has been proven to work with either 0.1% TFA or 0.25% FA (vol/vol) mobile phase. Addition of a guard column like a Phenomenex SecurityGuard AQ C18 pre-column guard cartridge can be optionally used. These optional changes to the method have no impact on the method’s performance. |
| | + | The Secondary HPLC method uses a Restek Pinnacle II Biphenyl 5 µm (150 x 4.6 mm) or comparable column and is intended as a confirmatory method. Like the Primary method, this method can use an optional guard column and utilizes a gradient elution, shown in Table 3. |
| | + | |
| | + | For instruments equipped with a mass spectrometer (MS), a secondary MS method is available and was developed alongside the Primary UV method. The method was designed for use with a single quadrupole MS equipped with an atmospheric pressure chemical ionization (APCI) source, such as an Agilent 6120B. A majority of the analytes shown in Table 1 are amenable to this MS method, however nitroglycerine (which is covered extensively in USEPA method 8332) and 2-,3-, and 4-nitrotoluene compounds aren’t compatible with the MS method. MS method parameters are shown in Table 4. |
| | | | |
| − | Remedy selection for treatment of rinsates involves several key factors. It is critical that environmental practitioners have up-to-date technical and practical knowledge on the suitability of these remedial options for different site conditions, treatment volumes, PFAS composition (e.g., presence of precursors, co-contaminants), PFAS concentrations, safety considerations, potential for undesired byproducts (e.g., perchlorate, disinfection byproducts), and treatment costs (e.g., energy demand, capital costs, operational labor).
| + | ==Summary== |
| | + | The extraction methods and instrumental methods in this article build upon prior munitions analytical methods by adding new compounds, combining legacy and insensitive munitions analysis, and expanding usable sample matrices. These methods have been verified through extensive round robin testing and validation, and while the methods are somewhat challenging, they are crucial when simultaneous analysis of both insensitive and legacy munitions is needed. |
| | | | |
| | ==References== | | ==References== |
| Line 73: |
Line 59: |
| | | | |
| | ==See Also== | | ==See Also== |
| | + | *[https://serdp-estcp.mil/focusareas/9f7a342a-1b13-4ce5-bda0-d7693cf2b82d/uxo#subtopics SERDP/ESTCP Focus Areas – UXO – Munitions Constituents] |
| | + | *[https://denix.osd.mil/edqw/home/ Environmental Data Quality Workgroup] |
Munitions Constituents – Sample Extraction and Analytical Techniques
Munitions Constituents, including insensitive munitions IM), are a broad category of compounds and, in areas where manufactured or used, can be found in a variety of environmental matrices (waters, soil, and tissues). This presents an analytical challenge when a variety of these munitions are to be quantified. This article discusses sample extraction methods for each typical sample matrix (high level water, low level water, soil and tissue) as well as the accompanying HPLC-UV analytical method for 27 compounds of interest (legacy munitions, insensitive munitions, and surrogates).
Related Article(s):
Contributor(s):
Key Resource(s):
- Methods for simultaneous quantification of legacy and insensitive munition (IM) constituents in aqueous, soil/sediment, and tissue matrices[2]
Introduction
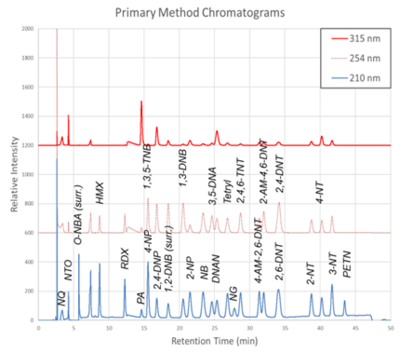
Figure 1. Primary Method labeled chromatograms
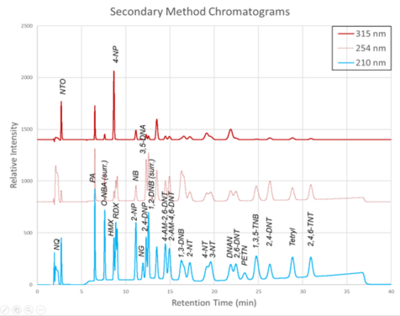
Figure 2. Secondary Method labeled chromatograms
The primary intention of the analytical methods presented here is to support the monitoring of legacy and insensitive munitions contamination on test and training ranges, however legacy and insensitive munitions often accompany each other at demilitarization facilities, manufacturing facilities, and other environmental sites. Energetic materials typically appear on ranges as small, solid particulates and due to their varying functional groups and polarities, can partition in various environmental compartments[3]. To ensure that contaminants are monitored and controlled at these sites and to sustainably manage them a variety of sample matrices (surface or groundwater, process waters, soil, and tissues) must be considered. (Process water refers to water used during industrial manufacturing or processing of legacy and insensitive munitions.) Furthermore, additional analytes must be added to existing methodologies as the usage of IM compounds changes and as new degradation compounds are identified. Of note, relatively new IM formulations containing NTO, DNAN, and NQ are seeing use in IMX-101, IMX-104, Pax-21 and Pax-41 (Table 1)[4][5].
Sampling procedures for legacy and insensitive munitions are identical and utilize multi-increment sampling procedures found in USEPA Method 8330B Appendix A[1]. Sample hold times, subsampling and quality control requirements are also unchanged. The key differences lie in the extraction methods and instrumental methods. Briefly, legacy munitions analysis of low concentration waters uses a single cartridge reverse phase SPE procedure, and acetonitrile (ACN) is used for both extraction and elution for aqueous and solid samples[1][6]. An isocratic separation via reversed-phase C-18 column with 50:50 methanol:water mobile phase or a C-8 column with 15:85 isopropanol:water mobile phase is used to separate legacy munitions[1]. While these procedures are sufficient for analysis of legacy munitions, alternative solvents, additional SPE cartridges, and a gradient elution are all required for the combined analysis of legacy and insensitive munitions.
Previously, analysis of legacy and insensitive munitions required multiple analytical techniques, however the methods presented here combine the two munitions categories resulting in an HPLC-UV method and accompanying extraction methods for a variety of common sample matrices. A secondary HPLC-UV method and a HPLC-MS method were also developed as confirmatory methods. The methods discussed in this article were validated extensively by single-blind round robin testing and subsequent statistical treatment as part of ESTCP ER19-5078. Wherever possible, the quality control criteria in the Department of Defense Quality Systems Manual for Environmental Laboratories were adhered to[7]. Analytes included in these methods are found in Table 1.
The chromatograms produced by the primary and secondary HPLC-UV methods are shown in Figure 1 and Figure 2, respectively. Chromatograms for each detector wavelength used are shown (315, 254, and 210 nm).
High Concentration Waters (> 1 ppm)
Aqueous samples suspected to contain the compounds of interest at concentrations detectable without any extraction or pre-concentration are suitable for analysis by direct injection. The method deviates from USEPA Method 8330B by adding a pH adjustment and use of MeOH rather than ACN for dilution[1]. The pH adjustment is needed to ensure method accuracy for ionic compounds (like NTO or PA) in basic samples. A solution of 1% HCl/MeOH is added to both acidify and dilute the samples to a final acid concentration of 0.5% (vol/vol) and a final solvent ratio of 1:1 MeOH/H2O. The direct injection samples are then ready for analysis.
Low Concentration Waters (< 1 ppm)
Aqueous samples suspected to contain the compounds of interest at low concentrations require extraction and pre-concentration using solid phase extraction (SPE). The SPE setup described here uses a triple cartridge setup shown in Figure 3. Briefly, the extraction procedure loads analytes of interest onto the cartridges in this order: StrataTM X, StrataTM X-A, and Envi-CarbTM. Then the cartridge order is reversed, and analytes are eluted via a two-step elution, resulting in 2 extracts (which are combined prior to analysis). Five milliliters of MeOH is used for the first elution, while 5 mL of acidified MeOH (2% HCl) is used for the second elution. The particular SPE cartridges used are noncritical so long as cartridge chemistries are comparable to those above.
Soils
Soil collection, storage, drying and grinding procedures are identical to the USEPA Method 8330B procedures[1]; however, the solvent extraction procedure differs in the number of sonication steps, sample mass and solvent used. A flow chart of the soil extraction procedure is shown in Figure 4. Soil masses of approximately 2 g and a sample to solvent ratio of 1:5 (g/mL) are used for soil extraction. The extraction is carried out in a sonication bath chilled below 20 ⁰C and is a two-part extraction, first extracting in MeOH (6 hours) followed by a second sonication in 1:1 MeOH:H2O solution (14 hours). The extracts are centrifuged, and the supernatant is filtered through a 0.45 μm PTFE disk filter.
The solvent volume should generally be 10 mL but if different soil masses are required, solvent volume should be 5 mL/g. The extraction results in 2 separate extracts (MeOH and MeOH:H2O) that are combined prior to analysis.
Tissues
Tissue matrices are extracted by 18-hour sonication using a ratio of 1 gram of wet tissue per 5 mL of MeOH. This extraction is performed in a sonication bath chilled below 20 ⁰C and the supernatant (MeOH) is filtered through a 0.45 μm PTFE disk filter.
Due to the complexity of tissue matrices, an additional tissue cleanup step, adapted from prior research, can be used to reduce interferences[8][2]. The cleanup procedure uses small scale chromatography columns prepared by loading 5 ¾” borosilicate pipettes with 0.2 g activated silica gel (100–200 mesh). The columns are wetted with 1 mL MeOH, which is allowed to fully elute and then discarded prior to loading with 1 mL of extract and collecting in a new amber vial. After the extract is loaded, a 1 mL aliquot of MeOH followed by a 1 mL aliquot of 2% HCL/MeOH is added. This results in a 3 mL silica treated tissue extract. This extract is vortexed and diluted to a final solvent ratio of 1:1 MeOH/H2O before analysis.
HPLC-UV and MS Methods
The Primary HPLC method uses a Phenomenex Synergi 4 µm Hydro-RP column (80Å, 250 x 4.6 mm), or comparable, and is based on both the HPLC method found in USEPA 8330B and previous work[1][8][2]. This separation relies on a reverse phase column and uses a gradient elution, shown in Table 2. Depending on the analyst’s needs and equipment availability, the method has been proven to work with either 0.1% TFA or 0.25% FA (vol/vol) mobile phase. Addition of a guard column like a Phenomenex SecurityGuard AQ C18 pre-column guard cartridge can be optionally used. These optional changes to the method have no impact on the method’s performance.
The Secondary HPLC method uses a Restek Pinnacle II Biphenyl 5 µm (150 x 4.6 mm) or comparable column and is intended as a confirmatory method. Like the Primary method, this method can use an optional guard column and utilizes a gradient elution, shown in Table 3.
For instruments equipped with a mass spectrometer (MS), a secondary MS method is available and was developed alongside the Primary UV method. The method was designed for use with a single quadrupole MS equipped with an atmospheric pressure chemical ionization (APCI) source, such as an Agilent 6120B. A majority of the analytes shown in Table 1 are amenable to this MS method, however nitroglycerine (which is covered extensively in USEPA method 8332) and 2-,3-, and 4-nitrotoluene compounds aren’t compatible with the MS method. MS method parameters are shown in Table 4.
Summary
The extraction methods and instrumental methods in this article build upon prior munitions analytical methods by adding new compounds, combining legacy and insensitive munitions analysis, and expanding usable sample matrices. These methods have been verified through extensive round robin testing and validation, and while the methods are somewhat challenging, they are crucial when simultaneous analysis of both insensitive and legacy munitions is needed.
References
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 United States Environmental Protection Agency (USEPA), 2006. EPA Method 8330B (SW-846) Nitroaromatics, Nitramines, and Nitrate Esters by High Performance Liquid Chromatography (HPLC), Revision 2. USEPA Website Method 8330B.pdf
- ^ 2.0 2.1 2.2 Crouch, R.A., Smith, J.C., Stromer, B.S., Hubley, C.T., Beal, S., Lotufo, G.R., Butler, A.D., Wynter, M.T., Russell, A.L., Coleman, J.G., Wayne, K.M., Clausen, J.L., Bednar, A.J., 2020. Methods for simultaneous determination of legacy and insensitive munition (IM) constituents in aqueous, soil/sediment, and tissue matrices. Talanta, 217, Article 121008. doi: 10.1016/j.talanta.2020.121008 Open Access Manuscript.pdf
- ^ Walsh, M.R., Temple, T., Bigl, M.F., Tshabalala, S.F., Mai, N. and Ladyman, M., 2017. Investigation of Energetic Particle Distribution from High‐Order Detonations of Munitions. Propellants, Explosives, Pyrotechnics, 42(8), pp. 932-941. doi: 10.1002/prep.201700089
- ^ Mainiero, C. 2015. Picatinny Employees Recognized for Insensitive Munitions. U.S. Army, Picatinny Arsenal Public Affairs. Open Access Press Release
- ^ Frem, D., 2022. A Review on IMX-101 and IMX-104 Melt-Cast Explosives: Insensitive Formulations for the Next-Generation Munition Systems. Propellants, Explosives, Pyrotechnics, 48(1), e202100312. doi: 10.1002/prep.202100312
- ^ United States Environmental Protection Agency (USEPA), 2007. EPA Method 3535A (SW-846) Solid-Phase Extraction (SPE), Revision 1. USEPA Website Method 3535A.pdf
- ^ US Department of Defense and US Department of Energy, 2021. Consolidated Quality Systems Manual (QSM) for Environmental Laboratories, Version 5.4. 387 pages. Free Download QSM Version 5.4.pdf
- ^ 8.0 8.1 Russell, A.L., Seiter, J.M., Coleman, J.G., Winstead, B., Bednar, A.J., 2014. Analysis of munitions constituents in IMX formulations by HPLC and HPLC-MS. Talanta, 128, pp. 524–530. doi: 10.1016/j.talanta.2014.02.013
See Also

