Difference between revisions of "User:Jhurley/sandbox"
(→Biochar) |
|||
| Line 1: | Line 1: | ||
| + | ==Remediation of Stormwater Runoff Contaminated by Munition Constituents== | ||
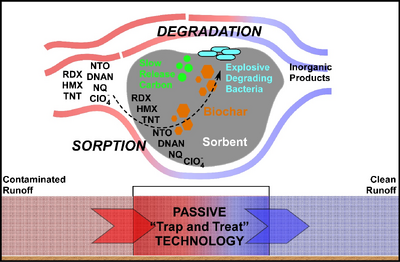
| + | Past and ongoing military operations have resulted in contamination of surface soil with [[Munitions Constituents | munition constituents (MC)]], which have human and environmental health impacts. These compounds can be transported off site via stormwater runoff during precipitation events. Technologies to “trap and treat” surface runoff before it enters downstream receiving bodies (e.g., streams, rivers, ponds) (see Figure 1), and which are compatible with ongoing range activities are needed. This article describes a passive and sustainable approach for effective management of munition constituents in stormwater runoff. | ||
| + | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | ||
| − | + | '''Related Article(s):''' | |
| − | + | *[[Munitions Constituents]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | ''' | + | '''Contributor:''' Mark E. Fuller |
| − | |||
| − | |||
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
| − | * | + | *SERDP Project ER19-1106: Development of Innovative Passive and Sustainable Treatment Technologies for Energetic Compounds in Surface Runoff on Active Ranges |
| − | |||
| − | == | + | ==Background== |
| − | [[File: | + | ===Surface Runoff Characteristics and Treatment Approaches=== |
| − | + | [[File: FullerFig1.png | thumb | 400 px | Figure 1. Conceptual model of passive trap and treat approach for MC removal from stormwater runoff]] | |
| − | + | During large precipitation events the rate of water deposition exceeds the rate of water infiltration, resulting in surface runoff (also called stormwater runoff). Surface characteristics including soil texture, presence of impermeable surfaces (natural and artificial), slope, and density and type of vegetation all influence the amount of surface runoff from a given land area. The use of passive systems such as retention ponds and biofiltration cells for treatment of surface runoff is well established for urban and roadway runoff. Treatment in those cases is typically achieved by directing runoff into and through a small constructed wetland, often at the outlet of a retention basin, or via filtration by directing runoff through a more highly engineered channel or vault containing the treatment materials. Filtration based technologies have proven to be effective for the removal of metals, organics, and suspended solids<ref>Sansalone, J.J., 1999. In-situ performance of a passive treatment system for metal source control. Water Science and Technology, 39(2), pp. 193-200. [https://doi.org/10.1016/S0273-1223(99)00023-2 doi: 10.1016/S0273-1223(99)00023-2]</ref><ref>Deletic, A., Fletcher, T.D., 2006. Performance of grass filters used for stormwater treatment—A field and modelling study. Journal of Hydrology, 317(3-4), pp. 261-275. [http://dx.doi.org/10.1016/j.jhydrol.2005.05.021 doi: 10.1016/j.jhydrol.2005.05.021]</ref><ref>Grebel, J.E., Charbonnet, J.A., Sedlak, D.L., 2016. Oxidation of organic contaminants by manganese oxide geomedia for passive urban stormwater treatment systems. Water Research, 88, pp. 481-491. [http://dx.doi.org/10.1016/j.watres.2015.10.019 doi: 10.1016/j.watres.2015.10.019]</ref><ref>Seelsaen, N., McLaughlan, R., Moore, S., Ball, J.E., Stuetz, R.M., 2006. Pollutant removal efficiency of alternative filtration media in stormwater treatment. Water Science and Technology, 54(6-7), pp. 299-305. [https://doi.org/10.2166/wst.2006.617 doi: 10.2166/wst.2006.617]</ref>. | |
| − | [[File: | + | ===Surface Runoff on Ranges=== |
| − | : | + | [[File: FullerFig2.png | thumb | 500 px | Figure 2. Conceptual illustration of munition constituent production and transport on military ranges. Mesoscale residues are qualitatively defined as being easily visible to the naked eye (e.g., from around 50 µm to multiple cm in size) and less likely to be transported by moving water. Microscale residues are defined as <50 µm down to below 1 µm, and more likely to be entrained in, and transported by, moving water as particulates. Blue arrows represent possible water flow paths and include both dissolved and solid phase energetics. The red vertical arrow represents the predominant energetics dissolution process in close proximity to the residues due to precipitation.]] |
| − | :: | + | Surface runoff represents a major potential mechanism through which energetics residues and related materials are transported off site from range soils to groundwater and surface water receptors (Figure 2). This process is particularly important for energetics that are water soluble (e.g., [[Wikipedia: Nitrotriazolone | NTO]] and [[Wikipedia: Nitroguanidine | NQ]]) or generate soluble daughter products (e.g., [[Wikipedia: 2,4-Dinitroanisole | DNAN]] and [[Wikipedia: TNT | TNT]]). While traditional MC such as [[Wikipedia: RDX | RDX]] and [[Wikipedia: HMX | HMX]] have limited aqueous solubility, they also exhibit recalcitrance to degrade under most natural conditions. RDX and [[Wikipedia: Perchlorate | perchlorate]] are frequent groundwater contaminants on military training ranges. While actual field measurements of energetics in surface runoff are limited, laboratory experiments have been performed to predict mobile energetics contamination levels based on soil mass loadings<ref>Cubello, F., Polyakov, V., Meding, S.M., Kadoya, W., Beal, S., Dontsova, K., 2024. Movement of TNT and RDX from composition B detonation residues in solution and sediment during runoff. Chemosphere, 350, Article 141023. [https://doi.org/10.1016/j.chemosphere.2023.141023 doi: 10.1016/j.chemosphere.2023.141023]</ref><ref>Karls, B., Meding, S.M., Li, L., Polyakov, V., Kadoya, W., Beal, S., Dontsova, K., 2023. A laboratory rill study of IMX-104 transport in overland flow. Chemosphere, 310, Article 136866. [https://doi.org/10.1016/j.chemosphere.2022.136866 doi: 10.1016/j.chemosphere.2022.136866] [[Media: KarlsEtAl2023.pdf | Open Access Article]]</ref><ref>Polyakov, V., Beal, S., Meding, S.M., Dontsova, K., 2025. Effect of gypsum on transport of IMX-104 constituents in overland flow under simulated rainfall. Journal of Environmental Quality, 54(1), pp. 191-203. [https://doi.org/10.1002/jeq2.20652 doi: 10.1002/jeq2.20652] [[Media: PolyakovEtAl2025.pdf | Open Access Article.pdf]]</ref><ref>Polyakov, V., Kadoya, W., Beal, S., Morehead, H., Hunt, E., Cubello, F., Meding, S.M., Dontsova, K., 2023. Transport of insensitive munitions constituents, NTO, DNAN, RDX, and HMX in runoff and sediment under simulated rainfall. Science of the Total Environment, 866, Article 161434. [https://doi.org/10.1016/j.scitotenv.2023.161434 doi: 10.1016/j.scitotenv.2023.161434] [[Media: PolyakovEtAl2023.pdf | Open Access Article.pdf]]</ref><ref>Price, R.A., Bourne, M., Price, C.L., Lindsay, J., Cole, J., 2011. Transport of RDX and TNT from Composition-B Explosive During Simulated Rainfall. In: Environmental Chemistry of Explosives and Propellant Compounds in Soils and Marine Systems: Distributed Source Characterization and Remedial Technologies. American Chemical Society, pp. 229-240. [https://doi.org/10.1021/bk-2011-1069.ch013 doi: 10.1021/bk-2011-1069.ch013]</ref>. For example, in a previous small study, MC were detected in surface runoff from an active live-fire range<ref>Fuller, M.E., 2015. Fate and Transport of Colloidal Energetic Residues. Department of Defense Strategic Environmental Research and Development Program (SERDP), Project ER-1689. [https://serdp-estcp.mil/projects/details/10760fd6-fb55-4515-a629-f93c555a92f0 Project Website] [[Media: ER-1689-FR.pdf | Final Report.pdf]]</ref>, and more recent sampling has detected MC in marsh surface water adjacent to the same installation (personal communication). Another recent report from Canada also detected RDX in both surface runoff and surface water at low part per billion levels in a survey of several military demolition sites<ref>Lapointe, M.-C., Martel, R., Diaz, E., 2017. A Conceptual Model of Fate and Transport Processes for RDX Deposited to Surface Soils of North American Active Demolition Sites. Journal of Environmental Quality, 46(6), pp. 1444-1454. [https://doi.org/10.2134/jeq2017.02.0069 doi: 10.2134/jeq2017.02.0069]</ref>. However, overall, data regarding the MC contaminant profile of surface runoff from ranges is very limited, and the possible presence of non-energetic constituents (e.g., metals, binders, plasticizers) in runoff has not been examined. Additionally, while energetics-contaminated surface runoff is an important concern, mitigation technologies specifically for surface runoff have not yet been developed and widely deployed in the field. To effectively capture and degrade MC and associated compounds that are present in surface runoff, novel treatment media are needed to sorb a broad range of energetic materials and to transform the retained compounds through abiotic and/or microbial processes. |
| − | :: | ||
| − | :: | ||
| − | :: | ||
| − | |||
| − | + | Surface runoff of organic and inorganic contaminants from live-fire ranges is a challenging issue for the Department of Defense (DoD). Potentially even more problematic is the fact that inputs to surface waters from large testing and training ranges typically originate from multiple sources, often encompassing hundreds of acres. No available technologies are currently considered effective for controlling non-point source energetics-laden surface runoff. While numerous technologies exist to treat collected explosives residues, contaminated soil and even groundwater, the decentralized nature and sheer volume of military range runoff have precluded the use of treatment technologies at full scale in the field. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ==Range Runoff Treatment Technology Components== |
| − | + | Based on the conceptual foundation of previous research into surface water runoff treatment for other contaminants, with a goal to “trap and treat” the target compounds, the following components were selected for inclusion in the technology developed to address range runoff contaminated with energetic compounds. | |
| − | + | ===Peat=== | |
| − | + | Previous research demonstrated that a peat-based system provided a natural and sustainable sorptive medium for organic explosives such as HMX, RDX, and TNT, allowing much longer residence times than predicted from hydraulic loading alone<ref>Fuller, M.E., Hatzinger, P.B., Rungkamol, D., Schuster, R.L., Steffan, R.J., 2004. Enhancing the attenuation of explosives in surface soils at military facilities: Combined sorption and biodegradation. Environmental Toxicology and Chemistry, 23(2), pp. 313-324. [https://doi.org/10.1897/03-187 doi: 10.1897/03-187]</ref><ref>Fuller, M.E., Lowey, J.M., Schaefer, C.E., Steffan, R.J., 2005. A Peat Moss-Based Technology for Mitigating Residues of the Explosives TNT, RDX, and HMX in Soil. Soil and Sediment Contamination: An International Journal, 14(4), pp. 373-385. [https://doi.org/10.1080/15320380590954097 doi: 10.1080/15320380590954097]</ref><ref name="FullerEtAl2009">Fuller, M.E., Schaefer, C.E., Steffan, R.J., 2009. Evaluation of a peat moss plus soybean oil (PMSO) technology for reducing explosive residue transport to groundwater at military training ranges under field conditions. Chemosphere, 77(8), pp. 1076-1083. [https://doi.org/10.1016/j.chemosphere.2009.08.044 doi: 10.1016/j.chemosphere.2009.08.044]</ref><ref>Hatzinger, P.B., Fuller, M.E., Rungkamol, D., Schuster, R.L., Steffan, R.J., 2004. Enhancing the attenuation of explosives in surface soils at military facilities: Sorption-desorption isotherms. Environmental Toxicology and Chemistry, 23(2), pp. 306-312. [https://doi.org/10.1897/03-186 doi: 10.1897/03-186]</ref><ref name="SchaeferEtAl2005">Schaefer, C.E., Fuller, M.E., Lowey, J.M., Steffan, R.J., 2005. Use of Peat Moss Amended with Soybean Oil for Mitigation of Dissolved Explosive Compounds Leaching into the Subsurface: Insight into Mass Transfer Mechanisms. Environmental Engineering Science, 22(3), pp. 337-349. [https://doi.org/10.1089/ees.2005.22.337 doi: 10.1089/ees.2005.22.337]</ref>. Peat moss represents a bioactive environment for treatment of the target contaminants. While the majority of the microbial reactions are aerobic due to the presence of measurable dissolved oxygen in the bulk solution, anaerobic reactions (including methanogenesis) can occur in microsites within the peat. The peat-based substrate acts not only as a long term electron donor as it degrades but also acts as a strong sorbent. This is important in intermittently loaded systems in which a large initial pulse of MC can be temporarily retarded on the peat matrix and then slowly degraded as they desorb<ref name="FullerEtAl2009"/><ref name="SchaeferEtAl2005"/>. This increased residence time enhances the biotransformation of energetics and promotes the immobilization and further degradation of breakdown products. Abiotic degradation reactions are also likely enhanced by association with the organic-rich peat (e.g., via electron shuttling reactions of [[Wikipedia: Humic substance | humics]])<ref>Roden, E.E., Kappler, A., Bauer, I., Jiang, J., Paul, A., Stoesser, R., Konishi, H., Xu, H., 2010. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nature Geoscience, 3, pp. 417-421. [https://doi.org/10.1038/ngeo870 doi: 10.1038/ngeo870]</ref>. | |
| − | == | + | ===Soybean Oil=== |
| − | + | Modeling has indicated that peat moss amended with crude soybean oil would significantly reduce the flux of dissolved TNT, RDX, and HMX through the vadose zone to groundwater compared to a non-treated soil (see [https://serdp-estcp.mil/projects/details/20e2f05c-fd50-4fd3-8451-ba73300c7531 ESTCP ER-200434]). The technology was validated in field soil plots, showing a greater than 500-fold reduction in the flux of dissolved RDX from macroscale Composition B detonation residues compared to a non-treated control plot<ref name="FullerEtAl2009"/>. Laboratory testing and modeling indicated that the addition of soybean oil increased the biotransformation rates of RDX and HMX at least 10-fold compared to rates observed with peat moss alone<ref name="SchaeferEtAl2005"/>. Subsequent experiments also demonstrated the effectiveness of the amended peat moss material for stimulating perchlorate transformation when added to a highly contaminated soil (Fuller et al., unpublished data). These previous findings clearly demonstrate the effectiveness of peat-based materials for mitigating transport of both organic and inorganic energetic compounds through soil to groundwater. | |
| + | |||
| + | ===Biochar=== | ||
| + | Recent reports have highlighted additional materials that, either alone, or in combination with electron donors such as peat moss and soybean oil, may further enhance the sorption and degradation of surface runoff contaminants, including both legacy energetics and [[Wikipedia: Insensitive_munition#Insensitive_high_explosives | insensitive high explosives (IHE)]]. For instance, [[Wikipedia: Biochar | biochar]], a type of black carbon, has been shown to not only sorb a wide range of organic and inorganic contaminants including MCs<ref>Ahmad, M., Rajapaksha, A.U., Lim, J.E., Zhang, M., Bolan, N., Mohan, D., Vithanage, M., Lee, S.S., Ok, Y.S., 2014. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, pp. 19-33. [https://doi.org/10.1016/j.chemosphere.2013.10.071 doi: 10.1016/j.chemosphere.2013.10.071]</ref><ref>Mohan, D., Sarswat, A., Ok, Y.S., Pittman, C.U., 2014. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent – A critical review. Bioresource Technology, 160, pp. 191-202. [https://doi.org/10.1016/j.biortech.2014.01.120 doi: 10.1016/j.biortech.2014.01.120]</ref><ref>Oh, S.-Y., Seo, Y.-D., Jeong, T.-Y., Kim, S.-D., 2018. Sorption of Nitro Explosives to Polymer/Biomass-Derived Biochar. Journal of Environmental Quality, 47(2), pp. 353-360. [https://doi.org/10.2134/jeq2017.09.0357 doi: 10.2134/jeq2017.09.0357]</ref><ref>Xie, T., Reddy, K.R., Wang, C., Yargicoglu, E., Spokas, K., 2015. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Critical Reviews in Environmental Science and Technology, 45(9), pp. 939-969. [https://doi.org/10.1080/10643389.2014.924180 doi: 10.1080/10643389.2014.924180]</ref>, but also to facilitate their degradation<ref>Oh, S.-Y., Cha, D.K., Kim, B.-J., Chiu, P.C., 2002. Effect of adsorption to elemental iron on the transformation of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine in solution. Environmental Toxicology and Chemistry, 21(7), pp. 1384-1389. [https://doi.org/10.1002/etc.5620210708 doi: 10.1002/etc.5620210708]</ref><ref>Ye, J., Chiu, P.C., 2006. Transport of Atomic Hydrogen through Graphite and its Reaction with Azoaromatic Compounds. Environmental Science and Technology, 40(12), pp. 3959-3964. [https://doi.org/10.1021/es060038x doi: 10.1021/es060038x]</ref><ref name="OhChiu2009">Oh, S.-Y., Chiu, P.C., 2009. Graphite- and Soot-Mediated Reduction of 2,4-Dinitrotoluene and Hexahydro-1,3,5-trinitro-1,3,5-triazine. Environmental Science and Technology, 43(18), pp. 6983-6988. [https://doi.org/10.1021/es901433m doi: 10.1021/es901433m]</ref><ref name="OhEtAl2013">Oh, S.-Y., Son, J.-G., Chiu, P.C., 2013. Biochar-mediated reductive transformation of nitro herbicides and explosives. Environmental Toxicology and Chemistry, 32(3), pp. 501-508. [https://doi.org/10.1002/etc.2087 doi: 10.1002/etc.2087] [[Media: OhEtAl2013.pdf | Open Access Article.pdf]]</ref><ref name="XuEtAl2010">Xu, W., Dana, K.E., Mitch, W.A., 2010. Black Carbon-Mediated Destruction of Nitroglycerin and RDX by Hydrogen Sulfide. Environmental Science and Technology, 44(16), pp. 6409-6415. [https://doi.org/10.1021/es101307n doi: 10.1021/es101307n]</ref><ref>Xu, W., Pignatello, J.J., Mitch, W.A., 2013. Role of Black Carbon Electrical Conductivity in Mediating Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) Transformation on Carbon Surfaces by Sulfides. Environmental Science and Technology, 47(13), pp. 7129-7136. [https://doi.org/10.1021/es4012367 doi: 10.1021/es4012367]</ref>. Depending on the source biomass and [[Wikipedia: Pyrolysis| pyrolysis]] conditions, biochar can possess a high [[Wikipedia: Specific surface area | specific surface area]] (on the order of several hundred m<small><sup>2</sup></small>/g)<ref>Zhang, J., You, C., 2013. Water Holding Capacity and Absorption Properties of Wood Chars. Energy and Fuels, 27(5), pp. 2643-2648. [https://doi.org/10.1021/ef4000769 doi: 10.1021/ef4000769]</ref><ref>Gray, M., Johnson, M.G., Dragila, M.I., Kleber, M., 2014. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass and Bioenergy, 61, pp. 196-205. [https://doi.org/10.1016/j.biombioe.2013.12.010 doi: 10.1016/j.biombioe.2013.12.010]</ref> and hence a high sorption capacity. Biochar and other black carbon also exhibit especially high affinity for [[Wikipedia: Nitro compound | nitroaromatic compounds (NACs)]] including TNT and 2,4-dinitrotoluene (DNT)<ref>Sander, M., Pignatello, J.J., 2005. Characterization of Charcoal Adsorption Sites for Aromatic Compounds: Insights Drawn from Single-Solute and Bi-Solute Competitive Experiments. Environmental Science and Technology, 39(6), pp. 1606-1615. [https://doi.org/10.1021/es049135l doi: 10.1021/es049135l]</ref><ref name="ZhuEtAl2005">Zhu, D., Kwon, S., Pignatello, J.J., 2005. Adsorption of Single-Ring Organic Compounds to Wood Charcoals Prepared Under Different Thermochemical Conditions. Environmental Science and Technology 39(11), pp. 3990-3998. [https://doi.org/10.1021/es050129e doi: 10.1021/es050129e]</ref><ref name="ZhuPignatello2005">Zhu, D., Pignatello, J.J., 2005. Characterization of Aromatic Compound Sorptive Interactions with Black Carbon (Charcoal) Assisted by Graphite as a Model. Environmental Science and Technology, 39(7), pp. 2033-2041. [https://doi.org/10.1021/es0491376 doi: 10.1021/es0491376]</ref>. This is due to the strong [[Wikipedia: Pi-interaction | ''π-π'' electron donor-acceptor interactions]] between electron-rich graphitic domains in black carbon and the electron-deficient aromatic ring of the NAC<ref name="ZhuEtAl2005"/><ref name="ZhuPignatello2005"/>. These characteristics make biochar a potentially effective, low cost, and sustainable sorbent for removing MC and other contaminants from surface runoff and retaining them for subsequent degradation ''in situ''. | ||
| + | |||
| + | Furthermore, black carbon such as biochar can promote abiotic and microbial transformation reactions by facilitating electron transfer. That is, biochar is not merely a passive sorbent for contaminants, but also a redox mediator for their degradation. Biochar can promote contaminant degradation through two different mechanisms: electron conduction and electron storage<ref>Sun, T., Levin, B.D.A., Guzman, J.J.L., Enders, A., Muller, D.A., Angenent, L.T., Lehmann, J., 2017. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nature Communications, 8, Article 14873. [https://doi.org/10.1038/ncomms14873 doi: 10.1038/ncomms14873] [[Media: SunEtAl2017.pdf | Open Access Article.pdf]]</ref>. | ||
| + | |||
| + | First, the microscopic graphitic regions in biochar can adsorb contaminants like NACs strongly, as noted above, and also conduct reducing equivalents such as electrons and atomic hydrogen to the sorbed contaminants, thus promoting their reductive degradation. This catalytic process has been demonstrated for TNT, DNT, RDX, HMX, and [[Wikipedia: Nitroglycerin | nitroglycerin]]<ref>Oh, S.-Y., Cha, D.K., Chiu, P.C., 2002. Graphite-Mediated Reduction of 2,4-Dinitrotoluene with Elemental Iron. Environmental Science and Technology, 36(10), pp. 2178-2184. [https://doi.org/10.1021/es011474g doi: 10.1021/es011474g]</ref><ref>Oh, S.-Y., Cha, D.K., Kim, B.J., Chiu, P.C., 2004. Reduction of Nitroglycerin with Elemental Iron: Pathway, Kinetics, and Mechanisms. Environmental Science and Technology, 38(13), pp. 3723-3730. [https://doi.org/10.1021/es0354667 doi: 10.1021/es0354667]</ref><ref>Oh, S.-Y., Cha, D.K., Kim, B.J., Chiu, P.C., 2005. Reductive transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine, octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, and methylenedinitramine with elemental iron. Environmental Toxicology and Chemistry, 24(11), pp. 2812-2819. [https://doi.org/10.1897/04-662R.1 doi: 10.1897/04-662R.1]</ref><ref name="OhChiu2009"/><ref name="XuEtAl2010"/> and is expected to occur also for IHE including DNAN and NTO. | ||
| + | |||
| + | Second, biochar contains in its structure abundant redox-facile functional groups such as [[Wikipedia: Quinone | quinones]] and [[Wikipedia: Hydroquinone | hydroquinones]], which are known to accept and donate electrons reversibly. Depending on the biomass and pyrolysis temperature, certain biochar can possess a rechargeable electron storage capacity (i.e., reversible electron accepting and donating capacity) on the order of several millimoles e<small><sup>–</sup></small>/g<ref>Klüpfel, L., Keiluweit, M., Kleber, M., Sander, M., 2014. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science and Technology, 48(10), pp. 5601-5611. [https://doi.org/10.1021/es500906d doi: 10.1021/es500906d]</ref><ref>Prévoteau, A., Ronsse, F., Cid, I., Boeckx, P., Rabaey, K., 2016. The electron donating capacity of biochar is dramatically underestimated. Scientific Reports, 6, Article 32870. [https://doi.org/10.1038/srep32870 doi: 10.1038/srep32870] [[Media: PrevoteauEtAl2016.pdf | Open Access Article.pdf]]</ref><ref>Xin, D., Xian, M., Chiu, P.C., 2018. Chemical methods for determining the electron storage capacity of black carbon. MethodsX, 5, pp. 1515-1520. [https://doi.org/10.1016/j.mex.2018.11.007 doi: 10.1016/j.mex.2018.11.007] [[Media: XinEtAl2018.pdf | Open Access Article.pdf]]</ref>. This means that when "charged", biochar can provide electrons for either abiotic or biotic degradation of reducible compounds such as MC. The abiotic reduction of DNT and RDX mediated by biochar has been demonstrated<ref name="OhEtAl2013"/> and similar reactions are expected to occur for DNAN and NTO as well. Recent studies have shown that the electron storage capacity of biochar is also accessible to microbes. For example, soil bacteria such as [[Wikipedia: Geobacter | ''Geobacter'']] and [[Wikipedia: Shewanella | ''Shewanella'']] species can utilize oxidized (or "discharged") biochar as an electron acceptor for the oxidation of organic substrates such as lactate and acetate<ref>Kappler, A., Wuestner, M.L., Ruecker, A., Harter, J., Halama, M., Behrens, S., 2014. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environmental Science and Technology Letters, 1(8), pp. 339-344. [https://doi.org/10.1021/ez5002209 doi: 10.1021/ez5002209]</ref><ref name="SaquingEtAl2016">Saquing, J.M., Yu, Y.-H., Chiu, P.C., 2016. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environmental Science and Technology Letters, 3(2), pp. 62-66. [https://doi.org/10.1021/acs.estlett.5b00354 doi: 10.1021/acs.estlett.5b00354]</ref> and reduced (or "charged") biochar as an electron donor for the reduction of nitrate<ref name="SaquingEtAl2016"/>. This is significant because, through microbial access of stored electrons in biochar, contaminants that do not sorb strongly to biochar can still be degraded. | ||
| + | |||
| + | Similar to nitrate, perchlorate and other relatively water-soluble energetic compounds (e.g., NTO and NQ) may also be similarly transformed using reduced biochar as an electron donor. Unlike other electron donors, biochar can be recharged through biodegradation of organic substrates<ref name="SaquingEtAl2016"/> and thus can serve as a long-lasting sorbent and electron repository in soil. Similar to peat moss, the high porosity and surface area of biochar not only facilitate contaminant sorption but also create anaerobic reducing microenvironments in its inner pores, where reductive degradation of energetic compounds can take place. | ||
| + | |||
| + | ===Other Sorbents=== | ||
| + | Chitin and unmodified cellulose were predicted by [[Wikipedia: Density functional theory | Density Functional Theory]] methods to be favorable for absorption of NTO and NQ, as well as the legacy explosives<ref>Todde, G., Jha, S.K., Subramanian, G., Shukla, M.K., 2018. Adsorption of TNT, DNAN, NTO, FOX7, and NQ onto Cellulose, Chitin, and Cellulose Triacetate. Insights from Density Functional Theory Calculations. Surface Science, 668, pp. 54-60. [https://doi.org/10.1016/j.susc.2017.10.004 doi: 10.1016/j.susc.2017.10.004] [[Media: ToddeEtAl2018.pdf | Open Access Manuscript.pdf]]</ref>. Cationized cellulosic materials (e.g., cotton, wood shavings) have been shown to effectively remove negatively charged energetics like perchlorate and NTO from solution<ref name="FullerEtAl2022">Fuller, M.E., Farquharson, E.M., Hedman, P.C., Chiu, P., 2022. Removal of munition constituents in stormwater runoff: Screening of native and cationized cellulosic sorbents for removal of insensitive munition constituents NTO, DNAN, and NQ, and legacy munition constituents HMX, RDX, TNT, and perchlorate. Journal of Hazardous Materials, 424(C), Article 127335. [https://doi.org/10.1016/j.jhazmat.2021.127335 doi: 10.1016/j.jhazmat.2021.127335] [[Media: FullerEtAl2022.pdf | Open Access Manuscript.pdf]]</ref>. A substantial body of work has shown that modified cellulosic biopolymers can also be effective sorbents for removing metals from solution<ref>Burba, P., Willmer, P.G., 1983. Cellulose: a biopolymeric sorbent for heavy-metal traces in waters. Talanta, 30(5), pp. 381-383. [https://doi.org/10.1016/0039-9140(83)80087-3 doi: 10.1016/0039-9140(83)80087-3]</ref><ref>Brown, P.A., Gill, S.A., Allen, S.J., 2000. Metal removal from wastewater using peat. Water Research, 34(16), pp. 3907-3916. [https://doi.org/10.1016/S0043-1354(00)00152-4 doi: 10.1016/S0043-1354(00)00152-4]</ref><ref>O’Connell, D.W., Birkinshaw, C., O’Dwyer, T.F., 2008. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresource Technology, 99(15), pp. 6709-6724. [https://doi.org/10.1016/j.biortech.2008.01.036 doi: 10.1016/j.biortech.2008.01.036]</ref><ref>Wan Ngah, W.S., Hanafiah, M.A.K.M., 2008. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresource Technology, 99(10), pp. 3935-3948. [https://doi.org/10.1016/j.biortech.2007.06.011 doi: 10.1016/j.biortech.2007.06.011]</ref> and therefore will also likely be applicable for some of the metals that may be found in surface runoff at firing ranges. | ||
| + | |||
| + | ==Technology Evaluation== | ||
| + | Based on the properties of the target munition constituents, a combination of materials was expected to yield the best results to facilitate the sorption and subsequent biotic and abiotic degradation of the contaminants. | ||
| + | |||
| + | ===Sorbents=== | ||
| + | {| class="wikitable" style="margin-right: 30px; margin-left: auto; float:left; text-align:center;" | ||
| + | |+Table 1. [[Wikipedia: Freundlich equation | Freundlich]] and [[Wikipedia: Langmuir adsorption model | Langmuir]] adsorption parameters for insensitive and legacy explosives | ||
| + | |- | ||
| + | ! rowspan="2" | Compound | ||
| + | ! colspan="5" | Freundlich | ||
| + | ! colspan="5" | Langmuir | ||
| + | |- | ||
| + | ! Parameter !! Peat !! CAT Pine !! CAT Burlap !! CAT Cotton !! Parameter !! Peat !! CAT Pine !! CAT Burlap !! CAT Cotton | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | HMX | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | 0.08 +/- 0.00 || -- || -- || -- | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | 0.29 +/- 0.04 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | 1.70 +/- 0.18 || -- || -- || -- | ||
| + | ! ''b'' (L/mg) | ||
| + | | 0.39 +/- 0.09 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.91 || -- || -- || -- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.93 || -- || -- || -- | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | RDX | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | 0.11 +/- 0.02 || -- || -- || -- | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | 0.38 +/- 0.05 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | 2.75 +/- 0.63 || -- || -- || -- | ||
| + | ! ''b'' (L/mg) | ||
| + | | 0.23 +/- 0.08 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.69 || -- || -- || -- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.69 || -- || -- || -- | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | TNT | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | 1.21 +/- 0.15 || 1.02 +/- 0.04 || 0.36 +/- 0.02 || -- | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | 3.63 +/- 0.18 || 1.26 +/- 0.06 || -- || -- | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | 2.78 +/- 0.67 || 4.01 +/- 0.44 || 1.59 +/- 0.09 || -- | ||
| + | ! ''b'' (L/mg) | ||
| + | | 0.89 +/- 0.13 || 0.76 +/- 0.10 || -- || -- | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.81 || 0.93 || 0.98 || -- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.97 || 0.97 || -- || -- | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | NTO | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | -- || 0.94 +/- 0.05 || 0.41 +/- 0.05 || 0.26 +/- 0.06 | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | -- || 4.07 +/- 0.26 || 1.29 +/- 0.12 || 0.83 +/- .015 | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | -- || 1.61 +/- 0.11 || 2.43 +/- 0.41 || 2.53 +/- 0.76 | ||
| + | ! ''b'' (L/mg) | ||
| + | | -- || 0.30 +/- 0.04 || 0.36 +/- 0.08 || 0.30 +/- 0.15 | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | -- || 0.97 || 0.82 || 0.57 | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | -- || 0.99 || 0.89 || 0.58 | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | DNAN | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | 0.38 +/- 0.05 || 0.01 +/- 0.01 || -- || -- | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | 2.57 +/- 0.33 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | 1.71 +/- 0.20 || 0.70 +/- 0.13 || -- || -- | ||
| + | ! ''b'' (L/mg) | ||
| + | | 0.13 +/- 0.03 || -- || -- || -- | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.89 || 0.76 || -- || -- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | 0.92 || -- || -- || -- | ||
| + | |- | ||
| + | | colspan="12" style="background-color:white;" | | ||
| + | |- | ||
| + | ! rowspan="3" | ClO<sub>4</sup> | ||
| + | ! ''K<sub>f</sub>'' | ||
| + | | -- || 1.54 +/- 0.06 || 0.53 +/- 0.03 || -- | ||
| + | ! ''q<sub>m</sub>'' (mg/g) | ||
| + | | -- || 3.63 +/- 0.18 || 1.26 +/- 0.06 || -- | ||
| + | |- | ||
| + | ! ''n'' | ||
| + | | -- || 2.42 +/- 0.16 || 2.42 +/- 0.26 || -- | ||
| + | ! ''b'' (L/mg) | ||
| + | | -- || 0.89 +/- 0.13 || 0.76 +/- 0.10 || -- | ||
| + | |- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | -- || 0.97 || 0.92 || -- | ||
| + | ! ''r<sup>2</sup>'' | ||
| + | | -- || 0.97 || 0.97 || -- | ||
| + | |- | ||
| + | | colspan="12" style="text-align:left; background-color:white;" |<small>Notes:</small><br /><big>'''--'''</big> <small>Indicates the algorithm failed to converge on the model fitting parameters, therefore there was no successful model fit.<br />'''CAT''' Indicates cationized material.</small> | ||
| + | |} | ||
| + | |||
| + | The materials screened included [[Wikipedia: Sphagnum | ''Sphagnum'' peat moss]], primarily for sorption of HMX, RDX, TNT, and DNAN, as well as [[Wikipedia: Cationization of cotton | cationized cellulosics]] for removal of perchlorate and NTO. The cationized cellulosics that were examined included: pine sawdust, pine shavings, aspen shavings, cotton linters (fine, silky fibers which adhere to cotton seeds after ginning), [[Wikipedia: Chitin | chitin]], [[Wikipedia: Chitosan | chitosan]], burlap (landscaping grade), [[Wikipedia: Coir | coconut coir]], raw cotton, raw organic cotton, cleaned raw cotton, cotton fabric, and commercially cationized fabrics. | ||
| + | |||
| + | As shown in Table 1<ref name="FullerEtAl2022"/>, batch sorption testing indicated that a combination of Sphagnum peat moss and cationized pine shavings provided good removal of both the neutral organic energetics (HMX, RDX, TNT, DNAN) as well as the negatively charged energetics (perchlorate, NTO). | ||
| + | |||
| + | ===Slow Release Carbon Sources=== | ||
| + | {| class="wikitable" style="margin-right: 30px; margin-left: auto; float:left; text-align:center;" | ||
| + | |+Table 2. Slow-release Carbon Sources | ||
| + | |- | ||
| + | ! Material !! Abbreviation !! Commercial Source !! Notes | ||
| + | |- | ||
| + | | polylactic acid || PLA6 || [https://www.goodfellow.com/usa?srsltid=AfmBOoqEiqIbrvWb1Hn1Bc090efBUUfg6V4N3Vrn6ytajHMJR-FG1Ez- Goodfellow] || high molecular weight thermoplastic polyester | ||
| + | |- | ||
| + | | polylactic acid || PLA80 || [https://www.goodfellow.com/usa?srsltid=AfmBOoqEiqIbrvWb1Hn1Bc090efBUUfg6V4N3Vrn6ytajHMJR-FG1Ez- Goodfellow] || low molecular weight thermoplastic polyester | ||
| + | |- | ||
| + | | polyhydroxybutyrate || PHB || [https://www.goodfellow.com/usa?srsltid=AfmBOoqEiqIbrvWb1Hn1Bc090efBUUfg6V4N3Vrn6ytajHMJR-FG1Ez- Goodfellow] || bacterial polyester | ||
| + | |- | ||
| + | | polycaprolactone || PCL || [https://www.sarchemlabs.com/?hsa_acc=4540346154&hsa_cam=20281343997&hsa_grp&hsa_ad&hsa_src=x&hsa_tgt&hsa_kw&hsa_mt&hsa_net=adwords&hsa_ver=3&gad_source=1&gad_campaignid=21209931835 Sarchem Labs] || biodegradable polyester | ||
| + | |- | ||
| + | | polybutylene succinate || BioPBS || [https://us.mitsubishi-chemical.com/company/performance-polymers/ Mitsubishi Chemical Performance Polymers] || compostable bio-based product | ||
| + | |- | ||
| + | | sucrose ester of fatty acids || SEFA SP10 || [https://www.sisterna.com/ Sisterna] || food and cosmetics additive | ||
| + | |- | ||
| + | | sucrose ester of fatty acids || SEFA SP70 || [https://www.sisterna.com/ Sisterna] || food and cosmetics additive | ||
| + | |} | ||
| + | |||
| + | A range of biopolymers widely used in the production of biodegradable plastics were screened for their ability to support aerobic and anoxic biodegradation of the target munition constituents. These compounds and their sources are listed in Table 2. | ||
| + | |||
| + | Multiple pure bacterial strains and mixed cultures were screened for their ability to utilize the solid biopolymers as a carbon source to support energetic compound transformation and degradation. Pure strains included the aerobic RDX degrader [[Wikipedia: Rhodococcus | ''Rhodococcus'']] species DN22 (DN22 henceforth)<ref name="ColemanEtAl1998">Coleman, N.V., Nelson, D.R., Duxbury, T., 1998. Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biology and Biochemistry, 30(8-9), pp. 1159-1167. [https://doi.org/10.1016/S0038-0717(97)00172-7 doi: 10.1016/S0038-0717(97)00172-7]</ref> and [[Wikipedia: Gordonia (bacterium)|''Gordonia'']] species KTR9 (KTR9 henceforth)<ref name="ColemanEtAl1998"/>, the anoxic RDX degrader [[Wikipedia: Pseudomonas fluorencens | ''Pseudomonas fluorencens'']] species I-C (I-C henceforth)<ref>Pak, J.W., Knoke, K.L., Noguera, D.R., Fox, B.G., Chambliss, G.H., 2000. Transformation of 2,4,6-Trinitrotoluene by Purified Xenobiotic Reductase B from Pseudomonas fluorescens I-C. Applied and Environmental Microbiology, 66(11), pp. 4742-4750. [https://doi.org/10.1128/AEM.66.11.4742-4750.2000 doi: 10.1128/AEM.66.11.4742-4750.2000] [[Media: PakEtAl2000.pdf | Open AccessArticle.pdf]]</ref><ref>Fuller, M.E., McClay, K., Hawari, J., Paquet, L., Malone, T.E., Fox, B.G., Steffan, R.J., 2009. Transformation of RDX and other energetic compounds by xenobiotic reductases XenA and XenB. Applied Microbiology and Biotechnology, 84, pp. 535-544. [https://doi.org/10.1007/s00253-009-2024-6 doi: 10.1007/s00253-009-2024-6] [[Media: FullerEtAl2009.pdf | Open Access Manuscript]]</ref>, and the aerobic NQ degrader [[Wikipedia: Pseudomonas | ''Pseudomonas extremaustralis'']] species NQ5 (NQ5 henceforth)<ref>Kim, J., Fuller, M.E., Hatzinger, P.B., Chu, K.-H., 2024. Isolation and characterization of nitroguanidine-degrading microorganisms. Science of the Total Environment, 912, Article 169184. [https://doi.org/10.1016/j.scitotenv.2023.169184 doi: 10.1016/j.scitotenv.2023.169184]</ref>. Anaerobic mixed cultures were obtained from a membrane bioreactor (MBR) degrading a mixture of six explosives (HMX, RDX, TNT, NTO, NQ, DNAN), as well as perchlorate and nitrate<ref name="FullerEtAl2023">Fuller, M.E., Hedman, P.C., Chu, K.-H., Webster, T.S., Hatzinger, P.B., 2023. Evaluation of a sequential anaerobic-aerobic membrane bioreactor system for treatment of traditional and insensitive munitions constituents. Chemosphere, 340, Article 139887. [https://doi.org/10.1016/j.chemosphere.2023.139887 doi: 10.1016/j.chemosphere.2023.139887]</ref>. The results indicated that the slow release carbon sources [[Wikipedia: Polyhydroxybutyrate | polyhydroxybutyrate (PHB)]], [[Wikipedia: Polycaprolactone | polycaprolactone (PCL)]], and [[Wikipedia: Polybutylene succinate | polybutylene succinate (BioPBS)]] were effective for supporting the biodegradation of the mixture of energetics. | ||
| + | |||
| + | ===Biochar=== | ||
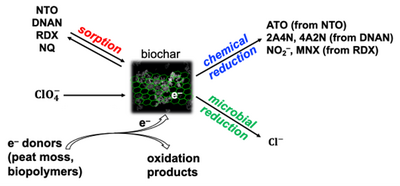
| + | [[File: FullerFig3.png | thumb | 400 px | Figure 3. Schematic of interactions between biochar and munitions constituents]] | ||
| + | The ability of biochar to sorb and abiotically reduce legacy and insensitive munition constituents, as well as biochar’s use as an electron donor for microbial biodegradation of energetic compounds was examined. Batch experiments indicated that biochar was a reasonable sorbent for some of the energetics (RDX, DNAN), but could also serve as both an electron acceptor and an electron donor to facilitate abiotic (RDX, DNAN, NTO) and biotic (perchlorate) degradation (Figure 3)<ref>Xin, D., Giron, J., Fuller, M.E., Chiu, P.C., 2022. Abiotic reduction of 3-nitro-1,2,4-triazol-5-one (NTO), DNAN, and RDX by wood-derived biochars through their rechargeable electron storage capacity. Environmental Science: Processes and Impacts, 24(2), pp. 316-329. [https://doi.org/10.1039/D1EM00447F doi: 10.1039/D1EM00447F] [[Media: XinEtAl2022.pdf | Open Access Manuscript.pdf]]</ref>. | ||
| + | |||
| + | ==Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Human Health== | ||
| + | Exposure pathways and effects for select PFAS are well understood, such that standard human health risk assessment approaches can be used to quantify risks for populations relevant to a site. Human health exposures via drinking water have been the focus in risk assessments and investigations at PFAS sites<ref>Post, G.B., Cohn, P.D., Cooper, K.R., 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environmental Research, 116, pp. 93-117. [https://doi.org/10.1016/j.envres.2012.03.007 doi: 10.1016/j.envres.2012.03.007]</ref><ref>Guelfo, J.L., Marlow, T., Klein, D.M., Savitz, D.A., Frickel, S., Crimi, M., Suuberg, E.M., 2018. Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities. Environmental Health Perspectives,126(6), 13 pages. [https://doi.org/10.1289/EHP2727 doi: 10.1289/EHP2727] [[Media: GuelfoEtAl2018.pdf | Open Access Article]]</ref>. Risk assessment approaches for PFAS in drinking water follow typical, well-established drinking water risk assessment approaches for chemicals as detailed in regulatory guidance documents for various jurisdictions. | ||
| + | |||
| + | Incidental exposures to soil and dusts for PFAS can occur during a variety of soil disturbance activities, such as gardening and digging, hand-to-mouth activities, and intrusive groundwork by industrial or construction workers. As detailed by the ITRC<ref name="ITRC2023"/>, many US states and USEPA have calculated risk-based screening levels for these soil and drinking water pathways (and many also include dermal exposures to soils) using well-established risk assessment guidance. | ||
| + | |||
| + | Field and laboratory studies have shown that some PFCAs and PFSAs bioaccumulate in fish and other aquatic life at rates that could result in relevant dietary PFAS exposures for consumers of fish and other seafood<ref>Martin, J.W., Mabury, S.A., Solomon, K.R., Muir, D.C., 2003. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 22(1), pp.189-195. [https://doi.org/10.1002/etc.5620220125 doi: 10.1002/etc.5620220125]</ref><ref>Martin, J.W., Mabury, S.A., Solomon, K.R., Muir, D.C., 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 22(1), pp.196-204. [https://doi.org/10.1002/etc.5620220126 doi: 10.1002/etc.5620220126]</ref><ref>Chen, F., Gong, Z., Kelly, B.C., 2016. Bioavailability and bioconcentration potential of perfluoroalkyl-phosphinic and -phosphonic acids in zebrafish (Danio rerio): Comparison to perfluorocarboxylates and perfluorosulfonates. Science of The Total Environment, 568, pp. 33-41. [https://doi.org/10.1016/j.scitotenv.2016.05.215 doi: 10.1016/j.scitotenv.2016.05.215]</ref><ref>Fang, S., Zhang, Y., Zhao, S., Qiang, L., Chen, M., Zhu, L., 2016. Bioaccumulation of per fluoroalkyl acids including the isomers of perfluorooctane sulfonate in carp (Cyprinus carpio) in a sediment/water microcosm. Environmental Toxicology and Chemistry, 35(12), pp. 3005-3013. [https://doi.org/10.1002/etc.3483 doi: 10.1002/etc.3483]</ref><ref>Bertin, D., Ferrari, B.J.D. Labadie, P., Sapin, A., Garric, J., Budzinski, H., Houde, M., Babut, M., 2014. Bioaccumulation of perfluoroalkyl compounds in midge (Chironomus riparius) larvae exposed to sediment. Environmental Pollution, 189, pp. 27-34. [https://doi.org/10.1016/j.envpol.2014.02.018 doi: 10.1016/j.envpol.2014.02.018]</ref><ref>Bertin, D., Labadie, P., Ferrari, B.J.D., Sapin, A., Garric, J., Geffard, O., Budzinski, H., Babut. M., 2016. Potential exposure routes and accumulation kinetics for poly- and perfluorinated alkyl compounds for a freshwater amphipod: Gammarus spp. (Crustacea). Chemosphere, 155, pp. 380-387. [https://doi.org/10.1016/j.chemosphere.2016.04.006 doi: 10.1016/j.chemosphere.2016.04.006]</ref><ref>Dai, Z., Xia, X., Guo, J., Jiang, X., 2013. Bioaccumulation and uptake routes of perfluoroalkyl acids in Daphnia magna. Chemosphere, 90(5), pp.1589-1596. [https://doi.org/10.1016/j.chemosphere.2012.08.026 doi: 10.1016/j.chemosphere.2012.08.026]</ref><ref>Prosser, R.S., Mahon, K., Sibley, P.K., Poirier, D., Watson-Leung, T. 2016. Bioaccumulation of perfluorinated carboxylates and sulfonates and polychlorinated biphenyls in laboratory-cultured Hexagenia spp., Lumbriculus variegatus and Pimephales promelas from field-collected sediments. Science of The Total Environment, 543(A), pp. 715-726. [https://doi.org/10.1016/j.scitotenv.2015.11.062 doi: 10.1016/j.scitotenv.2015.11.062]</ref><ref>Rich, C.D., Blaine, A.C., Hundal, L., Higgins, C., 2015. Bioaccumulation of Perfluoroalkyl Acids by Earthworms (Eisenia fetida) Exposed to Contaminated Soils. Environmental Science and Technology, 49(2) pp. 881-888. [https://doi.org/10.1021/es504152d doi: 10.1021/es504152d]</ref><ref>Muller, C.E., De Silva, A.O., Small, J., Williamson, M., Wang, X., Morris, A., Katz, S., Gamberg, M., Muir, D.C.G., 2011. Biomagnification of Perfluorinated Compounds in a Remote Terrestrial Food Chain: Lichen–Caribou–Wolf. Environmental Science and Technology, 45(20), pp. 8665-8673. [https://doi.org/10.1021/es201353v doi: 10.1021/es201353v]</ref>. In addition to fish, terrestrial wildlife can accumulate contaminants from impacted sites, resulting in potential exposures to consumers of wild game<ref name="ConderEtAl2021"/>. Additionally, exposures can occur though consumption of homegrown produce or agricultural products that originate from areas irrigated with PFAS-impacted groundwater, or that are amended with biosolids that contain PFAS, or that contain soils that were directly affected by PFAS releases<ref>Brown, J.B, Conder, J.M., Arblaster, J.A., Higgins, C.P., 2020. Assessing Human Health Risks from Per- and Polyfluoroalkyl Substance (PFAS)-Impacted Vegetable Consumption: A Tiered Modeling Approach. Environmental Science and Technology, 54(23), pp. 15202-15214. [https://doi.org/10.1021/acs.est.0c03411 doi: 10.1021/acs.est.0c03411] [[Media: BrownEtAl2020.pdf | Open Access Article]]</ref>. Multiple studies have found PFAS can be taken up by plants from soil porewater<ref>Blaine, A.C., Rich, C.D., Hundal, L.S., Lau, C., Mills, M.A., Harris, K.M., Higgins, C.P., 2013. Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environmental Science and Technology, 47(24), pp. 14062-14069. [https://doi.org/10.1021/es403094q doi: 10.1021/es403094q] [https://www.epa.gov/sites/production/files/2019-11/documents/508_pfascropuptake.pdf Free Download from epa.gov]</ref><ref>Blaine, A.C., Rich, C.D., Sedlacko, E.M., Hyland, K.C., Stushnoff, C., Dickenson, E.R.V., Higgins, C.P., 2014. Perfluoroalkyl Acid Uptake in Lettuce (Lactuca sativa) and Strawberry (Fragaria ananassa) Irrigated with Reclaimed Water. Environmental Science and Technology, 48(24), pp. 14361-14368. [https://doi.org/10.1021/es504150h doi: 10.1021/es504150h]</ref><ref>Ghisi, R., Vamerali, T., Manzetti, S., 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environmental Research, 169, pp. 326-341. [https://doi.org/10.1016/j.envres.2018.10.023 doi: 10.1016/j.envres.2018.10.023]</ref>, and livestock can accumulate PFAS from drinking water and/or feed<ref>van Asselt, E.D., Kowalczyk, J., van Eijkeren, J.C.H., Zeilmaker, M.J., Ehlers, S., Furst, P., Lahrssen-Wiederhold, M., van der Fels-Klerx, H.J., 2013. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chemistry, 141(2), pp.1489-1495. [https://doi.org/10.1016/j.foodchem.2013.04.035 doi: 10.1016/j.foodchem.2013.04.035]</ref>. Thus, when PFAS are present in surface water bodies where fishing or shellfish harvesting occurs or terrestrial areas where produce is grown or game is hunted, the bioaccumulation of PFAS into dietary items can be an important pathway for human exposure. | ||
| + | |||
| + | PFAAs such as PFOA and PFOS are not expected to volatilize from PFAS-impacted environmental media<ref name="USEPA2016a"/><ref name="USEPA2016b"/> such as soil and groundwater, which are the primary focus of most site-specific risk assessments. In contrast to non-volatile PFAAs, fluorotelomer alcohols (FTOHs) are among the more widely studied of the volatile PFAS. FTOHs are transient in the atmosphere with a lifetime of 20 days<ref>Ellis, D.A., Martin, J.W., De Silva, A.O., Mabury, S.A., Hurley, M.D., Sulbaek Andersen, M.P., Wallington, T.J., 2004. Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environmental Science and Technology, 38(12), pp. 3316-3321. [https://doi.org/10.1021/es049860w doi: 10.1021/es049860w]</ref>. At most AFFF sites under evaluation, AFFF releases have occurred many years before such that FTOH may no longer be present. As such, the current assumption is that volatile PFAS, such as FTOHs historically released at the site, will have transformed to stable, low-volatility PFAS, such as PFAAs in soil or groundwater, or will they have diffused to the outdoor atmosphere. There is no evidence that FTOHs or other volatile PFAS are persistent in groundwater or soils such that they present an indoor vapor intrusion pathway risk concern as observed for chlorinated solvents. Ongoing research continues for the vapor pathway<ref name="ITRC2023"/>. | ||
| + | |||
| + | General and site-specific human health exposure pathways and risk assessment methods as outlined by USEPA<ref>United States Environmental Protection Agency (USEPA), 1989. Risk Assessment Guidance for Superfund: Volume I, Human Health Evaluation Manual (Part A). Office of Solid Waste and Emergency Response, EPA/540/1-89/002. [https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=10001FQY.txt Free Download] [[Media: USEPA1989.pdf | Report.pdf]]</ref><ref name="USEPA1997">United States Environmental Protection Agency (USEPA), 1997. Ecological Risk Assessment Guidance for Superfund: Process for Designing and Conducting Ecological Risk Assessments, Interim Final. Office of Solid Waste and Emergency Response, EPA 540-R-97-006. [http://semspub.epa.gov/src/document/HQ/157941 Free Download] [[Media: EPA540-R-97-006.pdf | Report.pdf]]</ref> can be applied to PFAS risk assessments for which human health toxicity values have been developed. Additionally, for risk assessments with dietary exposures of PFAS, standard risk assessment food web modeling can be used to develop initial estimates of dietary concentrations which can be confirmed with site-specific tissue sampling programs. | ||
| + | |||
| + | ==Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Ecological== | ||
| + | Information available currently on exposures and effects of PFAS in ecological receptors indicate that the PFAS ecological risk issues at most sites are primarily associated with risks to vertebrate wildlife. Avian and mammalian wildlife are relatively sensitive to PFAS, and dietary intake via bioaccumulation in terrestrial and aquatic food webs can result in exposures that are dominated by the more accumulative PFAS<ref name="LarsonEtAl2018">Larson, E.S., Conder, J.M., Arblaster, J.A., 2018. Modeling avian exposures to perfluoroalkyl substances in aquatic habitats impacted by historical aqueous film forming foam releases. Chemosphere, 201, pp. 335-341. [https://doi.org/10.1016/j.chemosphere.2018.03.004 doi: 10.1016/j.chemosphere.2018.03.004]</ref><ref name="ConderEtAl2020"/><ref name="ZodrowEtAl2021a"/>. Direct toxicity to aquatic life (e.g., fish, pelagic life, benthic invertebrates, and aquatic plants) can occur from exposure to sediment and surface water at effected sites. For larger areas, surface water concentrations associated with adverse effects to aquatic life are generally higher than those that could result in adverse effects to aquatic-dependent wildlife. Soil invertebrates and plants are generally less sensitive, with risk-based concentrations in soil being much higher than those associated with potential effects to terrestrial wildlife<ref name="ZodrowEtAl2021a"/>. | ||
| + | |||
| + | Aquatic life are exposed to PFAS through direct exposure in surface water and sediment. Ecological risk assessment approaches for PFAS for aquatic life follow standard risk assessment approaches. The evaluation of potential risks for aquatic life with direct exposure to PFAS in environmental media relies on comparing concentrations in external exposure media to protective, media-specific benchmarks, including the aquatic life risk-based screening levels discussed above<ref name="ZodrowEtAl2021a"/><ref name="USEPA2024a">United States Environmental Protection Agency (USEPA), 2024. National Recommended Water Quality Criteria - Aquatic Life Criteria Table. [https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table USEPA Website]</ref>. | ||
| + | |||
| + | When an area at the point of PFAS release is an industrial setting which does not feature favorable habitats for terrestrial and aquatic-dependent wildlife, the transport mechanisms may allow PFAS to travel offsite. If offsite or downgradient areas contain ecological habitat, then PFAS transported to these areas are expected to pose the highest risk potential to wildlife, particularly those areas that feature aquatic habitat<ref>Ahrens, L., Bundschuh, M., 2014. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environmental Toxicology and Chemistry, 33(9), pp. 1921-1929. [https://doi.org/10.1002/etc.2663 doi: 10.1002/etc.2663] [[Media: AhrensBundschuh2014.pdf | Open Access Article]]</ref><ref name="LarsonEtAl2018"/>. | ||
| + | |||
| + | Wildlife receptors, specifically birds and mammals, are typically exposed to PFAS through uptake from dietary sources such as plants and invertebrates, along with direct soil ingestion during foraging activities. Dietary intake modeling typical for ecological risk assessments is the recommended approach for an evaluation of potential risks to wildlife species where PFAS exposure occurs primarily via dietary uptake from bioaccumulation pathways. Dietary intake modeling uses relevant exposure factors for each receptor group (terrestrial birds, terrestrial mammals, aquatic-dependent birds, and aquatic mammals) to determine a total daily intake (TDI) of PFAS via all potential exposure pathways. This approach requires determination of concentrations of PFAS in dietary items, which can be obtained by measuring PFAS in biota at sites or by using food web models to predict concentrations in biota using measured concentrations of PFAS in soil, sediment, or surface water. Food web models use bioaccumulation metrics such as bioaccumulation factors (BAFs) and biomagnification factors (BMFs) with measurements of PFAS in abiotic media to estimate concentrations in dietary items, including plants and benthic or pelagic invertebrates, to model wildlife exposure and calculate TDI. Once site-specific TDI values are calculated, they are compared to known TRVs identified from toxicity data with exposure doses associated with a lack of adverse effects (termed no observed adverse effect level [NOAEL]) or low adverse effects (termed lowest observed adverse effect level [LOAEL]), per standard risk assessment practice<ref name="USEPA1997"/>. | ||
| + | |||
| + | Recently, Conder ''et al.''<ref name="ConderEtAl2020"/>, Gobas ''et al.''<ref name="GobasEtAl2020"/>, and Zodrow ''et al.''<ref name="ZodrowEtAl2021a"/> compiled bioaccumulation modeling parameters and approaches for terrestrial and aquatic food web modeling of a variety of commonly detected PFAS at AFFF sites. There are also several sources of TRVs which can be relied upon for estimating TDI values<ref name="ConderEtAl2020"/><ref name="GobasEtAl2020"/><ref name="ZodrowEtAl2021a"/><ref>Newsted, J.L., Jones, P.D., Coady, K., Giesy, J.P., 2005. Avian Toxicity Reference Values for Perfluorooctane Sulfonate. Environmental Science and Technology, 39(23), pp. 9357-9362. [https://doi.org/10.1021/es050989v doi: 10.1021/es050989v]</ref><ref name="Suski2020"/>. In general, the highest risk for PFAS is expected for smaller insectivore and omnivore receptors (e.g., shrews and other small rodents, small nonmigratory birds), which tend to be lower in trophic level and spend more time foraging in small areas similar to or smaller in size than the impacted area. Compared to smaller, lower-trophic level organisms, larger mammalian and avian carnivores are expected to have lower exposures from site-specific PFAS sources because they forage over larger areas that may include areas that are not impacted, as compared to small organisms with small home ranges<ref name="LarsonEtAl2018"/><ref name="ConderEtAl2020"/><ref name="GobasEtAl2020"/><ref name="Suski2020"/><ref name="ZodrowEtAl2021a"/>. | ||
| + | |||
| + | Available information regarding PFAS exposure pathways and effects in aquatic life, terrestrial invertebrates and plants, as well as aquatic and terrestrial wildlife allow ecological risk assessment methods to be applied as outlined by USEPA<ref name="USEPA1997"/> to site-specific PFAS risk assessments. Additionally, food web modeling can be used in site-specific PFAS risk assessment to develop initial estimates of dietary concentrations for aquatic and terrestrial wildlife, which can be confirmed with tissue sampling programs at a site. | ||
| + | |||
| + | ==PFAS Risk Assessment Data Gaps== | ||
| + | There are a number of data gaps currently associated with PFAS risk assessment including the following: | ||
| + | *'''Unmeasured PFAS:''' There are a number of additional PFAS that we know little about and many PFAS that we are unable to quantify in the environment. The approach to dealing with the lack of information on the overwhelming number of PFAS is being debated; in the meantime, however, PFAS beyond PFOS and PFOA are being studied more, and this information will result in improved characterization of risks for other PFAS. | ||
| + | |||
| + | *'''Mixtures:''' Another major challenge in effects assessment for PFAS, for both human health risk assessments and environmental risk assessments, is understanding the potential importance of mixtures of PFAS. Considering the limited human health and ecological toxicity data available for just a few PFAS, the understanding of the relative toxicity, additivity, or synergistic effects of PFAS in mixtures is just beginning. | ||
| + | |||
| + | *'''Toxicity Data Gaps:''' For environmental risk assessments, some organisms such as reptiles and benthic invertebrates do not have toxicity data available. Benchmark or threshold concentrations of PFAS in environmental media intended to be protective of wildlife and aquatic organisms suffer from significant uncertainty in their derivation due to the limited number of species for which data are available. As species-specific data becomes available for more types of organisms, the accuracy of environmental risk assessments is likely to improve. | ||
==References== | ==References== | ||
| − | + | <references /> | |
| − | <references/> | ||
==See Also== | ==See Also== | ||
| − | + | [https://www.atsdr.cdc.gov/pfas/health-studies/index.html Agency for Toxic Substances and Disease Registry (ATSDR) PFAS Health Studies] | |
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 18:28, 27 October 2025
Remediation of Stormwater Runoff Contaminated by Munition Constituents
Past and ongoing military operations have resulted in contamination of surface soil with munition constituents (MC), which have human and environmental health impacts. These compounds can be transported off site via stormwater runoff during precipitation events. Technologies to “trap and treat” surface runoff before it enters downstream receiving bodies (e.g., streams, rivers, ponds) (see Figure 1), and which are compatible with ongoing range activities are needed. This article describes a passive and sustainable approach for effective management of munition constituents in stormwater runoff.
Contents
- 1 Remediation of Stormwater Runoff Contaminated by Munition Constituents
- 2 Background
- 3 Range Runoff Treatment Technology Components
- 4 Technology Evaluation
- 5 Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Human Health
- 6 Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Ecological
- 7 PFAS Risk Assessment Data Gaps
- 8 References
- 9 See Also
Related Article(s):
Contributor: Mark E. Fuller
Key Resource(s):
- SERDP Project ER19-1106: Development of Innovative Passive and Sustainable Treatment Technologies for Energetic Compounds in Surface Runoff on Active Ranges
Background
Surface Runoff Characteristics and Treatment Approaches
During large precipitation events the rate of water deposition exceeds the rate of water infiltration, resulting in surface runoff (also called stormwater runoff). Surface characteristics including soil texture, presence of impermeable surfaces (natural and artificial), slope, and density and type of vegetation all influence the amount of surface runoff from a given land area. The use of passive systems such as retention ponds and biofiltration cells for treatment of surface runoff is well established for urban and roadway runoff. Treatment in those cases is typically achieved by directing runoff into and through a small constructed wetland, often at the outlet of a retention basin, or via filtration by directing runoff through a more highly engineered channel or vault containing the treatment materials. Filtration based technologies have proven to be effective for the removal of metals, organics, and suspended solids[1][2][3][4].
Surface Runoff on Ranges
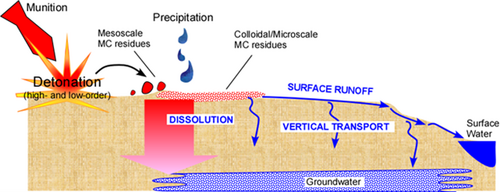
Surface runoff represents a major potential mechanism through which energetics residues and related materials are transported off site from range soils to groundwater and surface water receptors (Figure 2). This process is particularly important for energetics that are water soluble (e.g., NTO and NQ) or generate soluble daughter products (e.g., DNAN and TNT). While traditional MC such as RDX and HMX have limited aqueous solubility, they also exhibit recalcitrance to degrade under most natural conditions. RDX and perchlorate are frequent groundwater contaminants on military training ranges. While actual field measurements of energetics in surface runoff are limited, laboratory experiments have been performed to predict mobile energetics contamination levels based on soil mass loadings[5][6][7][8][9]. For example, in a previous small study, MC were detected in surface runoff from an active live-fire range[10], and more recent sampling has detected MC in marsh surface water adjacent to the same installation (personal communication). Another recent report from Canada also detected RDX in both surface runoff and surface water at low part per billion levels in a survey of several military demolition sites[11]. However, overall, data regarding the MC contaminant profile of surface runoff from ranges is very limited, and the possible presence of non-energetic constituents (e.g., metals, binders, plasticizers) in runoff has not been examined. Additionally, while energetics-contaminated surface runoff is an important concern, mitigation technologies specifically for surface runoff have not yet been developed and widely deployed in the field. To effectively capture and degrade MC and associated compounds that are present in surface runoff, novel treatment media are needed to sorb a broad range of energetic materials and to transform the retained compounds through abiotic and/or microbial processes.
Surface runoff of organic and inorganic contaminants from live-fire ranges is a challenging issue for the Department of Defense (DoD). Potentially even more problematic is the fact that inputs to surface waters from large testing and training ranges typically originate from multiple sources, often encompassing hundreds of acres. No available technologies are currently considered effective for controlling non-point source energetics-laden surface runoff. While numerous technologies exist to treat collected explosives residues, contaminated soil and even groundwater, the decentralized nature and sheer volume of military range runoff have precluded the use of treatment technologies at full scale in the field.
Range Runoff Treatment Technology Components
Based on the conceptual foundation of previous research into surface water runoff treatment for other contaminants, with a goal to “trap and treat” the target compounds, the following components were selected for inclusion in the technology developed to address range runoff contaminated with energetic compounds.
Peat
Previous research demonstrated that a peat-based system provided a natural and sustainable sorptive medium for organic explosives such as HMX, RDX, and TNT, allowing much longer residence times than predicted from hydraulic loading alone[12][13][14][15][16]. Peat moss represents a bioactive environment for treatment of the target contaminants. While the majority of the microbial reactions are aerobic due to the presence of measurable dissolved oxygen in the bulk solution, anaerobic reactions (including methanogenesis) can occur in microsites within the peat. The peat-based substrate acts not only as a long term electron donor as it degrades but also acts as a strong sorbent. This is important in intermittently loaded systems in which a large initial pulse of MC can be temporarily retarded on the peat matrix and then slowly degraded as they desorb[14][16]. This increased residence time enhances the biotransformation of energetics and promotes the immobilization and further degradation of breakdown products. Abiotic degradation reactions are also likely enhanced by association with the organic-rich peat (e.g., via electron shuttling reactions of humics)[17].
Soybean Oil
Modeling has indicated that peat moss amended with crude soybean oil would significantly reduce the flux of dissolved TNT, RDX, and HMX through the vadose zone to groundwater compared to a non-treated soil (see ESTCP ER-200434). The technology was validated in field soil plots, showing a greater than 500-fold reduction in the flux of dissolved RDX from macroscale Composition B detonation residues compared to a non-treated control plot[14]. Laboratory testing and modeling indicated that the addition of soybean oil increased the biotransformation rates of RDX and HMX at least 10-fold compared to rates observed with peat moss alone[16]. Subsequent experiments also demonstrated the effectiveness of the amended peat moss material for stimulating perchlorate transformation when added to a highly contaminated soil (Fuller et al., unpublished data). These previous findings clearly demonstrate the effectiveness of peat-based materials for mitigating transport of both organic and inorganic energetic compounds through soil to groundwater.
Biochar
Recent reports have highlighted additional materials that, either alone, or in combination with electron donors such as peat moss and soybean oil, may further enhance the sorption and degradation of surface runoff contaminants, including both legacy energetics and insensitive high explosives (IHE). For instance, biochar, a type of black carbon, has been shown to not only sorb a wide range of organic and inorganic contaminants including MCs[18][19][20][21], but also to facilitate their degradation[22][23][24][25][26][27]. Depending on the source biomass and pyrolysis conditions, biochar can possess a high specific surface area (on the order of several hundred m2/g)[28][29] and hence a high sorption capacity. Biochar and other black carbon also exhibit especially high affinity for nitroaromatic compounds (NACs) including TNT and 2,4-dinitrotoluene (DNT)[30][31][32]. This is due to the strong π-π electron donor-acceptor interactions between electron-rich graphitic domains in black carbon and the electron-deficient aromatic ring of the NAC[31][32]. These characteristics make biochar a potentially effective, low cost, and sustainable sorbent for removing MC and other contaminants from surface runoff and retaining them for subsequent degradation in situ.
Furthermore, black carbon such as biochar can promote abiotic and microbial transformation reactions by facilitating electron transfer. That is, biochar is not merely a passive sorbent for contaminants, but also a redox mediator for their degradation. Biochar can promote contaminant degradation through two different mechanisms: electron conduction and electron storage[33].
First, the microscopic graphitic regions in biochar can adsorb contaminants like NACs strongly, as noted above, and also conduct reducing equivalents such as electrons and atomic hydrogen to the sorbed contaminants, thus promoting their reductive degradation. This catalytic process has been demonstrated for TNT, DNT, RDX, HMX, and nitroglycerin[34][35][36][24][26] and is expected to occur also for IHE including DNAN and NTO.
Second, biochar contains in its structure abundant redox-facile functional groups such as quinones and hydroquinones, which are known to accept and donate electrons reversibly. Depending on the biomass and pyrolysis temperature, certain biochar can possess a rechargeable electron storage capacity (i.e., reversible electron accepting and donating capacity) on the order of several millimoles e–/g[37][38][39]. This means that when "charged", biochar can provide electrons for either abiotic or biotic degradation of reducible compounds such as MC. The abiotic reduction of DNT and RDX mediated by biochar has been demonstrated[25] and similar reactions are expected to occur for DNAN and NTO as well. Recent studies have shown that the electron storage capacity of biochar is also accessible to microbes. For example, soil bacteria such as Geobacter and Shewanella species can utilize oxidized (or "discharged") biochar as an electron acceptor for the oxidation of organic substrates such as lactate and acetate[40][41] and reduced (or "charged") biochar as an electron donor for the reduction of nitrate[41]. This is significant because, through microbial access of stored electrons in biochar, contaminants that do not sorb strongly to biochar can still be degraded.
Similar to nitrate, perchlorate and other relatively water-soluble energetic compounds (e.g., NTO and NQ) may also be similarly transformed using reduced biochar as an electron donor. Unlike other electron donors, biochar can be recharged through biodegradation of organic substrates[41] and thus can serve as a long-lasting sorbent and electron repository in soil. Similar to peat moss, the high porosity and surface area of biochar not only facilitate contaminant sorption but also create anaerobic reducing microenvironments in its inner pores, where reductive degradation of energetic compounds can take place.
Other Sorbents
Chitin and unmodified cellulose were predicted by Density Functional Theory methods to be favorable for absorption of NTO and NQ, as well as the legacy explosives[42]. Cationized cellulosic materials (e.g., cotton, wood shavings) have been shown to effectively remove negatively charged energetics like perchlorate and NTO from solution[43]. A substantial body of work has shown that modified cellulosic biopolymers can also be effective sorbents for removing metals from solution[44][45][46][47] and therefore will also likely be applicable for some of the metals that may be found in surface runoff at firing ranges.
Technology Evaluation
Based on the properties of the target munition constituents, a combination of materials was expected to yield the best results to facilitate the sorption and subsequent biotic and abiotic degradation of the contaminants.
Sorbents
| Compound | Freundlich | Langmuir | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Peat | CAT Pine | CAT Burlap | CAT Cotton | Parameter | Peat | CAT Pine | CAT Burlap | CAT Cotton | ||
| HMX | Kf | 0.08 +/- 0.00 | -- | -- | -- | qm (mg/g) | 0.29 +/- 0.04 | -- | -- | -- | |
| n | 1.70 +/- 0.18 | -- | -- | -- | b (L/mg) | 0.39 +/- 0.09 | -- | -- | -- | ||
| r2 | 0.91 | -- | -- | -- | r2 | 0.93 | -- | -- | -- | ||
| RDX | Kf | 0.11 +/- 0.02 | -- | -- | -- | qm (mg/g) | 0.38 +/- 0.05 | -- | -- | -- | |
| n | 2.75 +/- 0.63 | -- | -- | -- | b (L/mg) | 0.23 +/- 0.08 | -- | -- | -- | ||
| r2 | 0.69 | -- | -- | -- | r2 | 0.69 | -- | -- | -- | ||
| TNT | Kf | 1.21 +/- 0.15 | 1.02 +/- 0.04 | 0.36 +/- 0.02 | -- | qm (mg/g) | 3.63 +/- 0.18 | 1.26 +/- 0.06 | -- | -- | |
| n | 2.78 +/- 0.67 | 4.01 +/- 0.44 | 1.59 +/- 0.09 | -- | b (L/mg) | 0.89 +/- 0.13 | 0.76 +/- 0.10 | -- | -- | ||
| r2 | 0.81 | 0.93 | 0.98 | -- | r2 | 0.97 | 0.97 | -- | -- | ||
| NTO | Kf | -- | 0.94 +/- 0.05 | 0.41 +/- 0.05 | 0.26 +/- 0.06 | qm (mg/g) | -- | 4.07 +/- 0.26 | 1.29 +/- 0.12 | 0.83 +/- .015 | |
| n | -- | 1.61 +/- 0.11 | 2.43 +/- 0.41 | 2.53 +/- 0.76 | b (L/mg) | -- | 0.30 +/- 0.04 | 0.36 +/- 0.08 | 0.30 +/- 0.15 | ||
| r2 | -- | 0.97 | 0.82 | 0.57 | r2 | -- | 0.99 | 0.89 | 0.58 | ||
| DNAN | Kf | 0.38 +/- 0.05 | 0.01 +/- 0.01 | -- | -- | qm (mg/g) | 2.57 +/- 0.33 | -- | -- | -- | |
| n | 1.71 +/- 0.20 | 0.70 +/- 0.13 | -- | -- | b (L/mg) | 0.13 +/- 0.03 | -- | -- | -- | ||
| r2 | 0.89 | 0.76 | -- | -- | r2 | 0.92 | -- | -- | -- | ||
| ClO4 | Kf | -- | 1.54 +/- 0.06 | 0.53 +/- 0.03 | -- | qm (mg/g) | -- | 3.63 +/- 0.18 | 1.26 +/- 0.06 | -- | |
| n | -- | 2.42 +/- 0.16 | 2.42 +/- 0.26 | -- | b (L/mg) | -- | 0.89 +/- 0.13 | 0.76 +/- 0.10 | -- | ||
| r2 | -- | 0.97 | 0.92 | -- | r2 | -- | 0.97 | 0.97 | -- | ||
| Notes: -- Indicates the algorithm failed to converge on the model fitting parameters, therefore there was no successful model fit. CAT Indicates cationized material. | |||||||||||
The materials screened included Sphagnum peat moss, primarily for sorption of HMX, RDX, TNT, and DNAN, as well as cationized cellulosics for removal of perchlorate and NTO. The cationized cellulosics that were examined included: pine sawdust, pine shavings, aspen shavings, cotton linters (fine, silky fibers which adhere to cotton seeds after ginning), chitin, chitosan, burlap (landscaping grade), coconut coir, raw cotton, raw organic cotton, cleaned raw cotton, cotton fabric, and commercially cationized fabrics.
As shown in Table 1[43], batch sorption testing indicated that a combination of Sphagnum peat moss and cationized pine shavings provided good removal of both the neutral organic energetics (HMX, RDX, TNT, DNAN) as well as the negatively charged energetics (perchlorate, NTO).
Slow Release Carbon Sources
| Material | Abbreviation | Commercial Source | Notes |
|---|---|---|---|
| polylactic acid | PLA6 | Goodfellow | high molecular weight thermoplastic polyester |
| polylactic acid | PLA80 | Goodfellow | low molecular weight thermoplastic polyester |
| polyhydroxybutyrate | PHB | Goodfellow | bacterial polyester |
| polycaprolactone | PCL | Sarchem Labs | biodegradable polyester |
| polybutylene succinate | BioPBS | Mitsubishi Chemical Performance Polymers | compostable bio-based product |
| sucrose ester of fatty acids | SEFA SP10 | Sisterna | food and cosmetics additive |
| sucrose ester of fatty acids | SEFA SP70 | Sisterna | food and cosmetics additive |
A range of biopolymers widely used in the production of biodegradable plastics were screened for their ability to support aerobic and anoxic biodegradation of the target munition constituents. These compounds and their sources are listed in Table 2.
Multiple pure bacterial strains and mixed cultures were screened for their ability to utilize the solid biopolymers as a carbon source to support energetic compound transformation and degradation. Pure strains included the aerobic RDX degrader Rhodococcus species DN22 (DN22 henceforth)[48] and Gordonia species KTR9 (KTR9 henceforth)[48], the anoxic RDX degrader Pseudomonas fluorencens species I-C (I-C henceforth)[49][50], and the aerobic NQ degrader Pseudomonas extremaustralis species NQ5 (NQ5 henceforth)[51]. Anaerobic mixed cultures were obtained from a membrane bioreactor (MBR) degrading a mixture of six explosives (HMX, RDX, TNT, NTO, NQ, DNAN), as well as perchlorate and nitrate[52]. The results indicated that the slow release carbon sources polyhydroxybutyrate (PHB), polycaprolactone (PCL), and polybutylene succinate (BioPBS) were effective for supporting the biodegradation of the mixture of energetics.
Biochar
The ability of biochar to sorb and abiotically reduce legacy and insensitive munition constituents, as well as biochar’s use as an electron donor for microbial biodegradation of energetic compounds was examined. Batch experiments indicated that biochar was a reasonable sorbent for some of the energetics (RDX, DNAN), but could also serve as both an electron acceptor and an electron donor to facilitate abiotic (RDX, DNAN, NTO) and biotic (perchlorate) degradation (Figure 3)[53].
Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Human Health
Exposure pathways and effects for select PFAS are well understood, such that standard human health risk assessment approaches can be used to quantify risks for populations relevant to a site. Human health exposures via drinking water have been the focus in risk assessments and investigations at PFAS sites[54][55]. Risk assessment approaches for PFAS in drinking water follow typical, well-established drinking water risk assessment approaches for chemicals as detailed in regulatory guidance documents for various jurisdictions.
Incidental exposures to soil and dusts for PFAS can occur during a variety of soil disturbance activities, such as gardening and digging, hand-to-mouth activities, and intrusive groundwork by industrial or construction workers. As detailed by the ITRC[56], many US states and USEPA have calculated risk-based screening levels for these soil and drinking water pathways (and many also include dermal exposures to soils) using well-established risk assessment guidance.
Field and laboratory studies have shown that some PFCAs and PFSAs bioaccumulate in fish and other aquatic life at rates that could result in relevant dietary PFAS exposures for consumers of fish and other seafood[57][58][59][60][61][62][63][64][65][66]. In addition to fish, terrestrial wildlife can accumulate contaminants from impacted sites, resulting in potential exposures to consumers of wild game[67]. Additionally, exposures can occur though consumption of homegrown produce or agricultural products that originate from areas irrigated with PFAS-impacted groundwater, or that are amended with biosolids that contain PFAS, or that contain soils that were directly affected by PFAS releases[68]. Multiple studies have found PFAS can be taken up by plants from soil porewater[69][70][71], and livestock can accumulate PFAS from drinking water and/or feed[72]. Thus, when PFAS are present in surface water bodies where fishing or shellfish harvesting occurs or terrestrial areas where produce is grown or game is hunted, the bioaccumulation of PFAS into dietary items can be an important pathway for human exposure.
PFAAs such as PFOA and PFOS are not expected to volatilize from PFAS-impacted environmental media[73][74] such as soil and groundwater, which are the primary focus of most site-specific risk assessments. In contrast to non-volatile PFAAs, fluorotelomer alcohols (FTOHs) are among the more widely studied of the volatile PFAS. FTOHs are transient in the atmosphere with a lifetime of 20 days[75]. At most AFFF sites under evaluation, AFFF releases have occurred many years before such that FTOH may no longer be present. As such, the current assumption is that volatile PFAS, such as FTOHs historically released at the site, will have transformed to stable, low-volatility PFAS, such as PFAAs in soil or groundwater, or will they have diffused to the outdoor atmosphere. There is no evidence that FTOHs or other volatile PFAS are persistent in groundwater or soils such that they present an indoor vapor intrusion pathway risk concern as observed for chlorinated solvents. Ongoing research continues for the vapor pathway[56].
General and site-specific human health exposure pathways and risk assessment methods as outlined by USEPA[76][77] can be applied to PFAS risk assessments for which human health toxicity values have been developed. Additionally, for risk assessments with dietary exposures of PFAS, standard risk assessment food web modeling can be used to develop initial estimates of dietary concentrations which can be confirmed with site-specific tissue sampling programs.
Approaches for Evaluating Exposures and Effects in AFFF Site Environmental Risk Assessment: Ecological
Information available currently on exposures and effects of PFAS in ecological receptors indicate that the PFAS ecological risk issues at most sites are primarily associated with risks to vertebrate wildlife. Avian and mammalian wildlife are relatively sensitive to PFAS, and dietary intake via bioaccumulation in terrestrial and aquatic food webs can result in exposures that are dominated by the more accumulative PFAS[78][79][80]. Direct toxicity to aquatic life (e.g., fish, pelagic life, benthic invertebrates, and aquatic plants) can occur from exposure to sediment and surface water at effected sites. For larger areas, surface water concentrations associated with adverse effects to aquatic life are generally higher than those that could result in adverse effects to aquatic-dependent wildlife. Soil invertebrates and plants are generally less sensitive, with risk-based concentrations in soil being much higher than those associated with potential effects to terrestrial wildlife[80].
Aquatic life are exposed to PFAS through direct exposure in surface water and sediment. Ecological risk assessment approaches for PFAS for aquatic life follow standard risk assessment approaches. The evaluation of potential risks for aquatic life with direct exposure to PFAS in environmental media relies on comparing concentrations in external exposure media to protective, media-specific benchmarks, including the aquatic life risk-based screening levels discussed above[80][81].
When an area at the point of PFAS release is an industrial setting which does not feature favorable habitats for terrestrial and aquatic-dependent wildlife, the transport mechanisms may allow PFAS to travel offsite. If offsite or downgradient areas contain ecological habitat, then PFAS transported to these areas are expected to pose the highest risk potential to wildlife, particularly those areas that feature aquatic habitat[82][78].
Wildlife receptors, specifically birds and mammals, are typically exposed to PFAS through uptake from dietary sources such as plants and invertebrates, along with direct soil ingestion during foraging activities. Dietary intake modeling typical for ecological risk assessments is the recommended approach for an evaluation of potential risks to wildlife species where PFAS exposure occurs primarily via dietary uptake from bioaccumulation pathways. Dietary intake modeling uses relevant exposure factors for each receptor group (terrestrial birds, terrestrial mammals, aquatic-dependent birds, and aquatic mammals) to determine a total daily intake (TDI) of PFAS via all potential exposure pathways. This approach requires determination of concentrations of PFAS in dietary items, which can be obtained by measuring PFAS in biota at sites or by using food web models to predict concentrations in biota using measured concentrations of PFAS in soil, sediment, or surface water. Food web models use bioaccumulation metrics such as bioaccumulation factors (BAFs) and biomagnification factors (BMFs) with measurements of PFAS in abiotic media to estimate concentrations in dietary items, including plants and benthic or pelagic invertebrates, to model wildlife exposure and calculate TDI. Once site-specific TDI values are calculated, they are compared to known TRVs identified from toxicity data with exposure doses associated with a lack of adverse effects (termed no observed adverse effect level [NOAEL]) or low adverse effects (termed lowest observed adverse effect level [LOAEL]), per standard risk assessment practice[77].
Recently, Conder et al.[79], Gobas et al.[83], and Zodrow et al.[80] compiled bioaccumulation modeling parameters and approaches for terrestrial and aquatic food web modeling of a variety of commonly detected PFAS at AFFF sites. There are also several sources of TRVs which can be relied upon for estimating TDI values[79][83][80][84][85]. In general, the highest risk for PFAS is expected for smaller insectivore and omnivore receptors (e.g., shrews and other small rodents, small nonmigratory birds), which tend to be lower in trophic level and spend more time foraging in small areas similar to or smaller in size than the impacted area. Compared to smaller, lower-trophic level organisms, larger mammalian and avian carnivores are expected to have lower exposures from site-specific PFAS sources because they forage over larger areas that may include areas that are not impacted, as compared to small organisms with small home ranges[78][79][83][85][80].
Available information regarding PFAS exposure pathways and effects in aquatic life, terrestrial invertebrates and plants, as well as aquatic and terrestrial wildlife allow ecological risk assessment methods to be applied as outlined by USEPA[77] to site-specific PFAS risk assessments. Additionally, food web modeling can be used in site-specific PFAS risk assessment to develop initial estimates of dietary concentrations for aquatic and terrestrial wildlife, which can be confirmed with tissue sampling programs at a site.
PFAS Risk Assessment Data Gaps
There are a number of data gaps currently associated with PFAS risk assessment including the following:
- Unmeasured PFAS: There are a number of additional PFAS that we know little about and many PFAS that we are unable to quantify in the environment. The approach to dealing with the lack of information on the overwhelming number of PFAS is being debated; in the meantime, however, PFAS beyond PFOS and PFOA are being studied more, and this information will result in improved characterization of risks for other PFAS.
- Mixtures: Another major challenge in effects assessment for PFAS, for both human health risk assessments and environmental risk assessments, is understanding the potential importance of mixtures of PFAS. Considering the limited human health and ecological toxicity data available for just a few PFAS, the understanding of the relative toxicity, additivity, or synergistic effects of PFAS in mixtures is just beginning.
- Toxicity Data Gaps: For environmental risk assessments, some organisms such as reptiles and benthic invertebrates do not have toxicity data available. Benchmark or threshold concentrations of PFAS in environmental media intended to be protective of wildlife and aquatic organisms suffer from significant uncertainty in their derivation due to the limited number of species for which data are available. As species-specific data becomes available for more types of organisms, the accuracy of environmental risk assessments is likely to improve.
References
- ^ Sansalone, J.J., 1999. In-situ performance of a passive treatment system for metal source control. Water Science and Technology, 39(2), pp. 193-200. doi: 10.1016/S0273-1223(99)00023-2
- ^ Deletic, A., Fletcher, T.D., 2006. Performance of grass filters used for stormwater treatment—A field and modelling study. Journal of Hydrology, 317(3-4), pp. 261-275. doi: 10.1016/j.jhydrol.2005.05.021
- ^ Grebel, J.E., Charbonnet, J.A., Sedlak, D.L., 2016. Oxidation of organic contaminants by manganese oxide geomedia for passive urban stormwater treatment systems. Water Research, 88, pp. 481-491. doi: 10.1016/j.watres.2015.10.019
- ^ Seelsaen, N., McLaughlan, R., Moore, S., Ball, J.E., Stuetz, R.M., 2006. Pollutant removal efficiency of alternative filtration media in stormwater treatment. Water Science and Technology, 54(6-7), pp. 299-305. doi: 10.2166/wst.2006.617
- ^ Cubello, F., Polyakov, V., Meding, S.M., Kadoya, W., Beal, S., Dontsova, K., 2024. Movement of TNT and RDX from composition B detonation residues in solution and sediment during runoff. Chemosphere, 350, Article 141023. doi: 10.1016/j.chemosphere.2023.141023
- ^ Karls, B., Meding, S.M., Li, L., Polyakov, V., Kadoya, W., Beal, S., Dontsova, K., 2023. A laboratory rill study of IMX-104 transport in overland flow. Chemosphere, 310, Article 136866. doi: 10.1016/j.chemosphere.2022.136866 Open Access Article
- ^ Polyakov, V., Beal, S., Meding, S.M., Dontsova, K., 2025. Effect of gypsum on transport of IMX-104 constituents in overland flow under simulated rainfall. Journal of Environmental Quality, 54(1), pp. 191-203. doi: 10.1002/jeq2.20652 Open Access Article.pdf
- ^ Polyakov, V., Kadoya, W., Beal, S., Morehead, H., Hunt, E., Cubello, F., Meding, S.M., Dontsova, K., 2023. Transport of insensitive munitions constituents, NTO, DNAN, RDX, and HMX in runoff and sediment under simulated rainfall. Science of the Total Environment, 866, Article 161434. doi: 10.1016/j.scitotenv.2023.161434 Open Access Article.pdf
- ^ Price, R.A., Bourne, M., Price, C.L., Lindsay, J., Cole, J., 2011. Transport of RDX and TNT from Composition-B Explosive During Simulated Rainfall. In: Environmental Chemistry of Explosives and Propellant Compounds in Soils and Marine Systems: Distributed Source Characterization and Remedial Technologies. American Chemical Society, pp. 229-240. doi: 10.1021/bk-2011-1069.ch013
- ^ Fuller, M.E., 2015. Fate and Transport of Colloidal Energetic Residues. Department of Defense Strategic Environmental Research and Development Program (SERDP), Project ER-1689. Project Website Final Report.pdf
- ^ Lapointe, M.-C., Martel, R., Diaz, E., 2017. A Conceptual Model of Fate and Transport Processes for RDX Deposited to Surface Soils of North American Active Demolition Sites. Journal of Environmental Quality, 46(6), pp. 1444-1454. doi: 10.2134/jeq2017.02.0069
- ^ Fuller, M.E., Hatzinger, P.B., Rungkamol, D., Schuster, R.L., Steffan, R.J., 2004. Enhancing the attenuation of explosives in surface soils at military facilities: Combined sorption and biodegradation. Environmental Toxicology and Chemistry, 23(2), pp. 313-324. doi: 10.1897/03-187
- ^ Fuller, M.E., Lowey, J.M., Schaefer, C.E., Steffan, R.J., 2005. A Peat Moss-Based Technology for Mitigating Residues of the Explosives TNT, RDX, and HMX in Soil. Soil and Sediment Contamination: An International Journal, 14(4), pp. 373-385. doi: 10.1080/15320380590954097
- ^ 14.0 14.1 14.2 Fuller, M.E., Schaefer, C.E., Steffan, R.J., 2009. Evaluation of a peat moss plus soybean oil (PMSO) technology for reducing explosive residue transport to groundwater at military training ranges under field conditions. Chemosphere, 77(8), pp. 1076-1083. doi: 10.1016/j.chemosphere.2009.08.044
- ^ Hatzinger, P.B., Fuller, M.E., Rungkamol, D., Schuster, R.L., Steffan, R.J., 2004. Enhancing the attenuation of explosives in surface soils at military facilities: Sorption-desorption isotherms. Environmental Toxicology and Chemistry, 23(2), pp. 306-312. doi: 10.1897/03-186
- ^ 16.0 16.1 16.2 Schaefer, C.E., Fuller, M.E., Lowey, J.M., Steffan, R.J., 2005. Use of Peat Moss Amended with Soybean Oil for Mitigation of Dissolved Explosive Compounds Leaching into the Subsurface: Insight into Mass Transfer Mechanisms. Environmental Engineering Science, 22(3), pp. 337-349. doi: 10.1089/ees.2005.22.337
- ^ Roden, E.E., Kappler, A., Bauer, I., Jiang, J., Paul, A., Stoesser, R., Konishi, H., Xu, H., 2010. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nature Geoscience, 3, pp. 417-421. doi: 10.1038/ngeo870
- ^ Ahmad, M., Rajapaksha, A.U., Lim, J.E., Zhang, M., Bolan, N., Mohan, D., Vithanage, M., Lee, S.S., Ok, Y.S., 2014. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, pp. 19-33. doi: 10.1016/j.chemosphere.2013.10.071
- ^ Mohan, D., Sarswat, A., Ok, Y.S., Pittman, C.U., 2014. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent – A critical review. Bioresource Technology, 160, pp. 191-202. doi: 10.1016/j.biortech.2014.01.120
- ^ Oh, S.-Y., Seo, Y.-D., Jeong, T.-Y., Kim, S.-D., 2018. Sorption of Nitro Explosives to Polymer/Biomass-Derived Biochar. Journal of Environmental Quality, 47(2), pp. 353-360. doi: 10.2134/jeq2017.09.0357
- ^ Xie, T., Reddy, K.R., Wang, C., Yargicoglu, E., Spokas, K., 2015. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Critical Reviews in Environmental Science and Technology, 45(9), pp. 939-969. doi: 10.1080/10643389.2014.924180
- ^ Oh, S.-Y., Cha, D.K., Kim, B.-J., Chiu, P.C., 2002. Effect of adsorption to elemental iron on the transformation of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine in solution. Environmental Toxicology and Chemistry, 21(7), pp. 1384-1389. doi: 10.1002/etc.5620210708
- ^ Ye, J., Chiu, P.C., 2006. Transport of Atomic Hydrogen through Graphite and its Reaction with Azoaromatic Compounds. Environmental Science and Technology, 40(12), pp. 3959-3964. doi: 10.1021/es060038x
- ^ 24.0 24.1 Oh, S.-Y., Chiu, P.C., 2009. Graphite- and Soot-Mediated Reduction of 2,4-Dinitrotoluene and Hexahydro-1,3,5-trinitro-1,3,5-triazine. Environmental Science and Technology, 43(18), pp. 6983-6988. doi: 10.1021/es901433m
- ^ 25.0 25.1 Oh, S.-Y., Son, J.-G., Chiu, P.C., 2013. Biochar-mediated reductive transformation of nitro herbicides and explosives. Environmental Toxicology and Chemistry, 32(3), pp. 501-508. doi: 10.1002/etc.2087 Open Access Article.pdf
- ^ 26.0 26.1 Xu, W., Dana, K.E., Mitch, W.A., 2010. Black Carbon-Mediated Destruction of Nitroglycerin and RDX by Hydrogen Sulfide. Environmental Science and Technology, 44(16), pp. 6409-6415. doi: 10.1021/es101307n
- ^ Xu, W., Pignatello, J.J., Mitch, W.A., 2013. Role of Black Carbon Electrical Conductivity in Mediating Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) Transformation on Carbon Surfaces by Sulfides. Environmental Science and Technology, 47(13), pp. 7129-7136. doi: 10.1021/es4012367
- ^ Zhang, J., You, C., 2013. Water Holding Capacity and Absorption Properties of Wood Chars. Energy and Fuels, 27(5), pp. 2643-2648. doi: 10.1021/ef4000769
- ^ Gray, M., Johnson, M.G., Dragila, M.I., Kleber, M., 2014. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass and Bioenergy, 61, pp. 196-205. doi: 10.1016/j.biombioe.2013.12.010
- ^ Sander, M., Pignatello, J.J., 2005. Characterization of Charcoal Adsorption Sites for Aromatic Compounds: Insights Drawn from Single-Solute and Bi-Solute Competitive Experiments. Environmental Science and Technology, 39(6), pp. 1606-1615. doi: 10.1021/es049135l
- ^ 31.0 31.1 Zhu, D., Kwon, S., Pignatello, J.J., 2005. Adsorption of Single-Ring Organic Compounds to Wood Charcoals Prepared Under Different Thermochemical Conditions. Environmental Science and Technology 39(11), pp. 3990-3998. doi: 10.1021/es050129e
- ^ 32.0 32.1 Zhu, D., Pignatello, J.J., 2005. Characterization of Aromatic Compound Sorptive Interactions with Black Carbon (Charcoal) Assisted by Graphite as a Model. Environmental Science and Technology, 39(7), pp. 2033-2041. doi: 10.1021/es0491376
- ^ Sun, T., Levin, B.D.A., Guzman, J.J.L., Enders, A., Muller, D.A., Angenent, L.T., Lehmann, J., 2017. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nature Communications, 8, Article 14873. doi: 10.1038/ncomms14873 Open Access Article.pdf
- ^ Oh, S.-Y., Cha, D.K., Chiu, P.C., 2002. Graphite-Mediated Reduction of 2,4-Dinitrotoluene with Elemental Iron. Environmental Science and Technology, 36(10), pp. 2178-2184. doi: 10.1021/es011474g
- ^ Oh, S.-Y., Cha, D.K., Kim, B.J., Chiu, P.C., 2004. Reduction of Nitroglycerin with Elemental Iron: Pathway, Kinetics, and Mechanisms. Environmental Science and Technology, 38(13), pp. 3723-3730. doi: 10.1021/es0354667
- ^ Oh, S.-Y., Cha, D.K., Kim, B.J., Chiu, P.C., 2005. Reductive transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine, octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, and methylenedinitramine with elemental iron. Environmental Toxicology and Chemistry, 24(11), pp. 2812-2819. doi: 10.1897/04-662R.1
- ^ Klüpfel, L., Keiluweit, M., Kleber, M., Sander, M., 2014. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science and Technology, 48(10), pp. 5601-5611. doi: 10.1021/es500906d
- ^ Prévoteau, A., Ronsse, F., Cid, I., Boeckx, P., Rabaey, K., 2016. The electron donating capacity of biochar is dramatically underestimated. Scientific Reports, 6, Article 32870. doi: 10.1038/srep32870 Open Access Article.pdf
- ^ Xin, D., Xian, M., Chiu, P.C., 2018. Chemical methods for determining the electron storage capacity of black carbon. MethodsX, 5, pp. 1515-1520. doi: 10.1016/j.mex.2018.11.007 Open Access Article.pdf
- ^ Kappler, A., Wuestner, M.L., Ruecker, A., Harter, J., Halama, M., Behrens, S., 2014. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environmental Science and Technology Letters, 1(8), pp. 339-344. doi: 10.1021/ez5002209
- ^ 41.0 41.1 41.2 Saquing, J.M., Yu, Y.-H., Chiu, P.C., 2016. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environmental Science and Technology Letters, 3(2), pp. 62-66. doi: 10.1021/acs.estlett.5b00354
- ^ Todde, G., Jha, S.K., Subramanian, G., Shukla, M.K., 2018. Adsorption of TNT, DNAN, NTO, FOX7, and NQ onto Cellulose, Chitin, and Cellulose Triacetate. Insights from Density Functional Theory Calculations. Surface Science, 668, pp. 54-60. doi: 10.1016/j.susc.2017.10.004 Open Access Manuscript.pdf
- ^ 43.0 43.1 Fuller, M.E., Farquharson, E.M., Hedman, P.C., Chiu, P., 2022. Removal of munition constituents in stormwater runoff: Screening of native and cationized cellulosic sorbents for removal of insensitive munition constituents NTO, DNAN, and NQ, and legacy munition constituents HMX, RDX, TNT, and perchlorate. Journal of Hazardous Materials, 424(C), Article 127335. doi: 10.1016/j.jhazmat.2021.127335 Open Access Manuscript.pdf
- ^ Burba, P., Willmer, P.G., 1983. Cellulose: a biopolymeric sorbent for heavy-metal traces in waters. Talanta, 30(5), pp. 381-383. doi: 10.1016/0039-9140(83)80087-3
- ^ Brown, P.A., Gill, S.A., Allen, S.J., 2000. Metal removal from wastewater using peat. Water Research, 34(16), pp. 3907-3916. doi: 10.1016/S0043-1354(00)00152-4
- ^ O’Connell, D.W., Birkinshaw, C., O’Dwyer, T.F., 2008. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresource Technology, 99(15), pp. 6709-6724. doi: 10.1016/j.biortech.2008.01.036
- ^ Wan Ngah, W.S., Hanafiah, M.A.K.M., 2008. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresource Technology, 99(10), pp. 3935-3948. doi: 10.1016/j.biortech.2007.06.011
- ^ 48.0 48.1 Coleman, N.V., Nelson, D.R., Duxbury, T., 1998. Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biology and Biochemistry, 30(8-9), pp. 1159-1167. doi: 10.1016/S0038-0717(97)00172-7
- ^ Pak, J.W., Knoke, K.L., Noguera, D.R., Fox, B.G., Chambliss, G.H., 2000. Transformation of 2,4,6-Trinitrotoluene by Purified Xenobiotic Reductase B from Pseudomonas fluorescens I-C. Applied and Environmental Microbiology, 66(11), pp. 4742-4750. doi: 10.1128/AEM.66.11.4742-4750.2000 Open AccessArticle.pdf
- ^ Fuller, M.E., McClay, K., Hawari, J., Paquet, L., Malone, T.E., Fox, B.G., Steffan, R.J., 2009. Transformation of RDX and other energetic compounds by xenobiotic reductases XenA and XenB. Applied Microbiology and Biotechnology, 84, pp. 535-544. doi: 10.1007/s00253-009-2024-6 Open Access Manuscript
- ^ Kim, J., Fuller, M.E., Hatzinger, P.B., Chu, K.-H., 2024. Isolation and characterization of nitroguanidine-degrading microorganisms. Science of the Total Environment, 912, Article 169184. doi: 10.1016/j.scitotenv.2023.169184
- ^ Fuller, M.E., Hedman, P.C., Chu, K.-H., Webster, T.S., Hatzinger, P.B., 2023. Evaluation of a sequential anaerobic-aerobic membrane bioreactor system for treatment of traditional and insensitive munitions constituents. Chemosphere, 340, Article 139887. doi: 10.1016/j.chemosphere.2023.139887
- ^ Xin, D., Giron, J., Fuller, M.E., Chiu, P.C., 2022. Abiotic reduction of 3-nitro-1,2,4-triazol-5-one (NTO), DNAN, and RDX by wood-derived biochars through their rechargeable electron storage capacity. Environmental Science: Processes and Impacts, 24(2), pp. 316-329. doi: 10.1039/D1EM00447F Open Access Manuscript.pdf
- ^ Post, G.B., Cohn, P.D., Cooper, K.R., 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environmental Research, 116, pp. 93-117. doi: 10.1016/j.envres.2012.03.007
- ^ Guelfo, J.L., Marlow, T., Klein, D.M., Savitz, D.A., Frickel, S., Crimi, M., Suuberg, E.M., 2018. Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities. Environmental Health Perspectives,126(6), 13 pages. doi: 10.1289/EHP2727 Open Access Article
- ^ 56.0 56.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedITRC2023 - ^ Martin, J.W., Mabury, S.A., Solomon, K.R., Muir, D.C., 2003. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 22(1), pp.189-195. doi: 10.1002/etc.5620220125
- ^ Martin, J.W., Mabury, S.A., Solomon, K.R., Muir, D.C., 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 22(1), pp.196-204. doi: 10.1002/etc.5620220126
- ^ Chen, F., Gong, Z., Kelly, B.C., 2016. Bioavailability and bioconcentration potential of perfluoroalkyl-phosphinic and -phosphonic acids in zebrafish (Danio rerio): Comparison to perfluorocarboxylates and perfluorosulfonates. Science of The Total Environment, 568, pp. 33-41. doi: 10.1016/j.scitotenv.2016.05.215
- ^ Fang, S., Zhang, Y., Zhao, S., Qiang, L., Chen, M., Zhu, L., 2016. Bioaccumulation of per fluoroalkyl acids including the isomers of perfluorooctane sulfonate in carp (Cyprinus carpio) in a sediment/water microcosm. Environmental Toxicology and Chemistry, 35(12), pp. 3005-3013. doi: 10.1002/etc.3483
- ^ Bertin, D., Ferrari, B.J.D. Labadie, P., Sapin, A., Garric, J., Budzinski, H., Houde, M., Babut, M., 2014. Bioaccumulation of perfluoroalkyl compounds in midge (Chironomus riparius) larvae exposed to sediment. Environmental Pollution, 189, pp. 27-34. doi: 10.1016/j.envpol.2014.02.018
- ^ Bertin, D., Labadie, P., Ferrari, B.J.D., Sapin, A., Garric, J., Geffard, O., Budzinski, H., Babut. M., 2016. Potential exposure routes and accumulation kinetics for poly- and perfluorinated alkyl compounds for a freshwater amphipod: Gammarus spp. (Crustacea). Chemosphere, 155, pp. 380-387. doi: 10.1016/j.chemosphere.2016.04.006
- ^ Dai, Z., Xia, X., Guo, J., Jiang, X., 2013. Bioaccumulation and uptake routes of perfluoroalkyl acids in Daphnia magna. Chemosphere, 90(5), pp.1589-1596. doi: 10.1016/j.chemosphere.2012.08.026
- ^ Prosser, R.S., Mahon, K., Sibley, P.K., Poirier, D., Watson-Leung, T. 2016. Bioaccumulation of perfluorinated carboxylates and sulfonates and polychlorinated biphenyls in laboratory-cultured Hexagenia spp., Lumbriculus variegatus and Pimephales promelas from field-collected sediments. Science of The Total Environment, 543(A), pp. 715-726. doi: 10.1016/j.scitotenv.2015.11.062
- ^ Rich, C.D., Blaine, A.C., Hundal, L., Higgins, C., 2015. Bioaccumulation of Perfluoroalkyl Acids by Earthworms (Eisenia fetida) Exposed to Contaminated Soils. Environmental Science and Technology, 49(2) pp. 881-888. doi: 10.1021/es504152d
- ^ Muller, C.E., De Silva, A.O., Small, J., Williamson, M., Wang, X., Morris, A., Katz, S., Gamberg, M., Muir, D.C.G., 2011. Biomagnification of Perfluorinated Compounds in a Remote Terrestrial Food Chain: Lichen–Caribou–Wolf. Environmental Science and Technology, 45(20), pp. 8665-8673. doi: 10.1021/es201353v
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedConderEtAl2021 - ^ Brown, J.B, Conder, J.M., Arblaster, J.A., Higgins, C.P., 2020. Assessing Human Health Risks from Per- and Polyfluoroalkyl Substance (PFAS)-Impacted Vegetable Consumption: A Tiered Modeling Approach. Environmental Science and Technology, 54(23), pp. 15202-15214. doi: 10.1021/acs.est.0c03411 Open Access Article
- ^ Blaine, A.C., Rich, C.D., Hundal, L.S., Lau, C., Mills, M.A., Harris, K.M., Higgins, C.P., 2013. Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environmental Science and Technology, 47(24), pp. 14062-14069. doi: 10.1021/es403094q Free Download from epa.gov
- ^ Blaine, A.C., Rich, C.D., Sedlacko, E.M., Hyland, K.C., Stushnoff, C., Dickenson, E.R.V., Higgins, C.P., 2014. Perfluoroalkyl Acid Uptake in Lettuce (Lactuca sativa) and Strawberry (Fragaria ananassa) Irrigated with Reclaimed Water. Environmental Science and Technology, 48(24), pp. 14361-14368. doi: 10.1021/es504150h
- ^ Ghisi, R., Vamerali, T., Manzetti, S., 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environmental Research, 169, pp. 326-341. doi: 10.1016/j.envres.2018.10.023
- ^ van Asselt, E.D., Kowalczyk, J., van Eijkeren, J.C.H., Zeilmaker, M.J., Ehlers, S., Furst, P., Lahrssen-Wiederhold, M., van der Fels-Klerx, H.J., 2013. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chemistry, 141(2), pp.1489-1495. doi: 10.1016/j.foodchem.2013.04.035
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedUSEPA2016a - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedUSEPA2016b - ^ Ellis, D.A., Martin, J.W., De Silva, A.O., Mabury, S.A., Hurley, M.D., Sulbaek Andersen, M.P., Wallington, T.J., 2004. Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environmental Science and Technology, 38(12), pp. 3316-3321. doi: 10.1021/es049860w
- ^ United States Environmental Protection Agency (USEPA), 1989. Risk Assessment Guidance for Superfund: Volume I, Human Health Evaluation Manual (Part A). Office of Solid Waste and Emergency Response, EPA/540/1-89/002. Free Download Report.pdf
- ^ 77.0 77.1 77.2 United States Environmental Protection Agency (USEPA), 1997. Ecological Risk Assessment Guidance for Superfund: Process for Designing and Conducting Ecological Risk Assessments, Interim Final. Office of Solid Waste and Emergency Response, EPA 540-R-97-006. Free Download Report.pdf
- ^ 78.0 78.1 78.2 Larson, E.S., Conder, J.M., Arblaster, J.A., 2018. Modeling avian exposures to perfluoroalkyl substances in aquatic habitats impacted by historical aqueous film forming foam releases. Chemosphere, 201, pp. 335-341. doi: 10.1016/j.chemosphere.2018.03.004
- ^ 79.0 79.1 79.2 79.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedConderEtAl2020 - ^ 80.0 80.1 80.2 80.3 80.4 80.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedZodrowEtAl2021a - ^ United States Environmental Protection Agency (USEPA), 2024. National Recommended Water Quality Criteria - Aquatic Life Criteria Table. USEPA Website
- ^ Ahrens, L., Bundschuh, M., 2014. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environmental Toxicology and Chemistry, 33(9), pp. 1921-1929. doi: 10.1002/etc.2663 Open Access Article
- ^ 83.0 83.1 83.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedGobasEtAl2020 - ^ Newsted, J.L., Jones, P.D., Coady, K., Giesy, J.P., 2005. Avian Toxicity Reference Values for Perfluorooctane Sulfonate. Environmental Science and Technology, 39(23), pp. 9357-9362. doi: 10.1021/es050989v
- ^ 85.0 85.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedSuski2020
See Also
Agency for Toxic Substances and Disease Registry (ATSDR) PFAS Health Studies